Gaining Ground in the Race Against Antibiotic Resistance
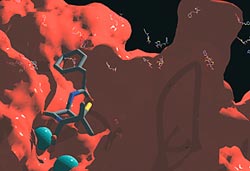
In this close-up of the metallo-beta-lactamase enzyme’s active site (in red), the two essential metal ions (blue spheres) and a beta-lactam antibiotic (colored sticks) are highlighted. The activity of this enzyme has been optimized by in vitro evolution resulting from exposure to large amounts of antibiotics. Over the black background (on top), a large number of antibiotic molecules are depicted. Image: Pablo E. Tomatis
Antibiotic resistance has put humans in an escalating ’arms race’ with infectious bacteria, as scientists try to develop new antibiotics faster than the bacteria can evolve new resistance strategies. But now, researchers have a new strategy that may give them a leg up in the race—reproducing in the lab the natural evolution of the bacterial enzymes that confer resistance.
A team of scientists in Argentina and Mexico identified mutations that increased the efficiency of a bacterial enzyme that renders penicillin and cephalosporin antibiotics useless. The results could lead to more effective enzyme inhibitors by giving drug designers a sneak peek at the next generation of resistance.
“We were mimicking what is going on in the doctor’s clinic, putting selection pressure on the enzyme by giving higher doses of antibiotic.”
Alejandro J. Vila
Alejandro Vila, a Howard Hughes Medical Institute international research scholar, and colleagues at the University of Rosario’s Institute of Molecular and Cellular Biology in Argentina and at the Biotechnology Institute of the National Autonomous University of Mexico report their findings in the early online edition of the Proceedings of the National Academy of Sciences the week of September 19, 2005.
Staying one step ahead of resistance with new antibiotics and treatments for infections is a huge challenge because bacteria evolve quickly to evade them. When the scientists introduced random mutations into the gene for a bacterial resistance enzyme and grew the bacteria on increasing concentrations of antibiotics, it took only a few days of test tube evolution to increase drug resistance. Eventually, they found four mutations in the evolved enzyme that allowed the bacteria to survive on a drug dose 64 times higher than the dose that kills bacteria hosting the un-evolved enzyme.
“We were mimicking what is going on in the doctor’s clinic—putting selection pressure on the enzyme by giving higher doses of antibiotic,” said Vila. “The only ones to survive will be those that have an enzyme that can work more efficiently.”
The researchers conducted their experiments using a drug called cephalexin, a member of the widely prescribed cephalosporin class of antibiotics. These drugs and the penicillins, which share a common chemical backbone called the â-lactam ring, work by disrupting the bacterial cell wall. Bacteria have evolved enzymes called â-lactamases, which chop the â-lactam ring in half, inactivating the drugs. An inhibitor for one type of lactamase has already been marketed as part of a ’package drug’ with amoxicillin to fight resistance.
But the lactamase enzyme that Vila’s group studied is in a different class that is causing an emerging problem around the world. This class, the metallo-â-lactamases, is more threatening, said Vila, because it is effective against a broader spectrum of antibiotics, such as carbapenems. However, it also represents a younger set of enzymes that are still evolving, and that enabled the scientists to observe that evolution in fast-forward.
The group used a lactamase gene from the Bacillus cereus soil bacteria and tested it in the laboratory strain E. coli. The gene is very similar to lactamase genes found in disease-causing bacteria such as Pseudomonas and Acinetobacter—common culprits in resistant, hard-to-treat hospital infections. And it is almost identical to a lactamase gene found in Bacillus anthracis, which causes anthrax.
Together, the four mutations identified by the group increased the enzyme’s efficiency at inactivating cephalexin seven-fold. The mutations influenced the enzyme’s active site, where the chopping of antibiotic molecules takes place. One of the mutations has already been found in nature, in a lactamase from Pseudomonas.
In some cases, there is a tradeoff associated with antibiotic resistance: the bacteria’s success in fighting a particular antibiotic can cause it to lose efficiency in inactivating other antibiotics. But that was not the case here.
“This evolved enzyme works better against cephalexin and with the same efficiency on other antibiotics, as well,” said Vila. In fact, the mutant enzyme inactivated seven other cephalosporins as efficiently as or better than the original enzyme. “So it hasn’t lost anything, and the outcome is that the bacteria has increased its range of resistance. This is a huge concern in the clinic.”
To date, there are no known inhibitors of metallo-â-lactamases, but directed evolution could help in their design, Vila said, by giving drug makers a reliable prediction of what the next generation of enzymes will look like. “Since we were able to reproduce the natural evolution in the test tube, you could generate a more efficient lactamase to use as a target, so that your inhibitor would be one step ahead.”
This would give science an edge in the resistance race, and it might help slow the vicious cycle enough to develop antibiotics impervious to lactamases.
Media Contact
More Information:
http://www.hhmi.orgAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















