Genomics reveals mechanism of heat resistance in bacteria
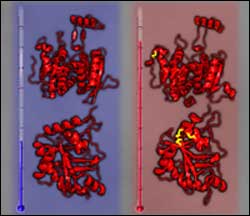
Thermophilic bacteria can thrive in extreme heat because their proteins have an abundance of disulfides (yellow, above), covalent bonds between sulfur atoms that improve stability and likely boost heat-tolerance. (Yeates et al.)
Warm-blooded creatures maintain a relatively stable body temperature that cannot tolerate the stress of intense heat (or cold). When it’s too hot proteins destabilize and degrade–in some cases, with fatal results. But some bacterial and archaeal organisms appear to defy nature (as we think of it) by flourishing in extremely high temperatures. The archaeal microbe Pyrobaculum aerophilum, for example–originally found in a boiling marine water hole in Italy–thrives at ~100 °C (212 °F).
Published online this week in the open-access journal PLoS Biology Todd Yeates and colleagues from UCLA have investigated the mechanisms that engineer this remarkable heat resistance. By way of an elegant analysis of publicly available genome sequence and protein structure data, they answer the question: how do these thermophilic bacteria and archaea manage to maintain active, stable proteins at such high temperatures? The authors found that proteins from P. aerophilum along with some other thermophiles have many disulfide bonds (covalent bonds between two spatially proximate cysteines), which are known to improve stability.
By mapping intracellular gene sequences from 199 prokaryote genomes onto sequence-related proteins with known three-dimensional structures, they produced structural models which revealed when disulfide bonds are likely to form. A bias was found for disulfides in a set of thermophilic genomes. To prove that these predictions really do form disulfide bonds, the authors solved the structure of one protein from P. aerophilum–which was indeed stabilized by three disulfide bonds.
Disulfide bonds are more commonly formed outside or between cells in multicellular organisms. The high numbers of bonds observed in these prokaryotes challenge our ideas of how disulfide bonds form. Given the difficulty for disulfides to form in such organisms, the authors investigated which proteins are present in the disulfide-rich organisms as compared with the proteins in other organisms (also known as phylogenetic profiling). They found a protein called protein disulfide oxidoreductase (PDO) present in all of the disulfide-rich thermophiles which is not seen in the other prokaryotes. As its name suggests, this protein likely plays a key role in the formation of disulfides in these heat-tolerant bugs.
Yeates and colleagues have considerably advanced our understanding of how proteins withstand and function at high temperatures via stabilizing disulfide bonds in these thermophilic organisms. Yet, since this correlation of extra disulfides and the PDO is not common to all thermophiles, it is likely that this is not the only method employed in heat resistance. Probably several different mechanisms are employed to enable thermophiles to flourish in extreme conditions. As the authors show here, genome sequence and structure data can help us to uncover these mechanisms.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

“Nanostitches” enable lighter and tougher composite materials
In research that may lead to next-generation airplanes and spacecraft, MIT engineers used carbon nanotubes to prevent cracking in multilayered composites. To save on fuel and reduce aircraft emissions, engineers…

Trash to treasure
Researchers turn metal waste into catalyst for hydrogen. Scientists have found a way to transform metal waste into a highly efficient catalyst to make hydrogen from water, a discovery that…

Real-time detection of infectious disease viruses
… by searching for molecular fingerprinting. A research team consisting of Professor Kyoung-Duck Park and Taeyoung Moon and Huitae Joo, PhD candidates, from the Department of Physics at Pohang University…





















