Plants, animals share molecular growth mechanisms
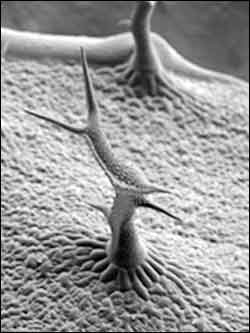
Malfunctioning genes that affect plant growth and development cause distortion in tiny hairs, called trichomes, that are found on most cells. Compared with normal plants, the trichome branch lengths are shorter and slightly twisted, and the base of the trichome is abnormally elongated and swollen. (Photo courtesy of Dan Szymanski)
A newly discovered plant protein complex that apparently switches on plants’ growth machinery, has opened a scientific toolbox to learn about both plant and animal development, according Purdue University scientists.
The protein complex triggers communication between molecules along a pathway that leads to the creation of long protein strings, called actin filaments, that are necessary for cellular growth, said Dan Szymanski, agronomy associate professor and lead author of the study. Knowledge of the biochemical reactions involved in this process eventually may allow researchers to design plants better able to protect themselves from insects and disease. “These genes and their proteins are required for normal development and for normal cell-to-cell adhesion,” Szymanski said. “They affect the growth of the whole plant and also the shape and size of types of cells in the plant.” Results of the study are published in the February issue of the journal The Plant Cell. “Perhaps by learning about this pathway for actin filament formation, we can engineer plant cells to grow in different ways or alter how cells respond to external stimuli so they can defend themselves against insect or fungal attacks,” Szymanski said.
A protein complex known as Actin Related Protein 2/3 (ARP2/3) is a cellular machine that controls formation of actin filaments, which are important for cell growth and movement. Actin filaments organize the inside of the cell and allow it to grow, and they determine where certain structures in a cell are positioned and how plants respond to gravity and light. Szymanski’s team used a deformed version of a common research plant, Arabidopsis thaliana, and specifically looked at small, hairlike structures that exist on most cells. They found that the shape and size of these hairs, or trichomes, readily show when genes affecting actin filaments are askew and causing altered growth. The researchers previously had learned that a large protein complex, known as WAVE, activated ARP2/3, but they didn’t know specifically which WAVE protein was the actual switch. Their latest research showed that a WAVE protein they’ve dubbed DISTORTED3 (DIS3) turns on APR 2/3, which in turn triggers formation of new, growing actin filaments.
Because some genes have survived through time as multicellular life evolved, they have been conserved in both plants and animals, Szymanski said. So, some of the plant proteins that comprise the ARP2/3 and the WAVE complexes are interchangeable with proteins in animals. Others proteins are not interchangeable, and Szymanski’s research team is delving into how this affects the growth process. “DIS3 has two ends that are common in both plant and animal proteins,” he said. “But DIS3 has a very large segment in the middle that is specific to plants. We’d like to know if this section is important and whether it regulates DIS3 or the whole WAVE complex.” For growth and development biochemical processes to proceed normally, activators such as ARP 2/3 are needed to trigger actin filaments’ formation and growth, Szymanski said. However, scientists don’t know the specific function of certain actin filaments. The molecular tools Szymanski’s research team developed will help scientists learn more about these functions in both plants and animals.
The other researchers on this study were Dipanwita Basu and Salah El-Din El-Essal, research assistants; postdoctoral students Jie Le, Chunhua Zhang and Gregore Koliantz; Eileen Malley, laboratory manager, all of the Department of Agronomy; and Shanjin Huang, postdoctoral student, and Christopher Staiger, professor, both of the Department of Biological Sciences. Staiger and Szymanski also are members of the Purdue Motility Group.
The Energy Biosciences Division of the Department of Energy, the USDA National Research Initiative and the Purdue Agricultural Research Program provided funding for this research.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Silicon Carbide Innovation Alliance to drive industrial-scale semiconductor work
Known for its ability to withstand extreme environments and high voltages, silicon carbide (SiC) is a semiconducting material made up of silicon and carbon atoms arranged into crystals that is…

New SPECT/CT technique shows impressive biomarker identification
…offers increased access for prostate cancer patients. A novel SPECT/CT acquisition method can accurately detect radiopharmaceutical biodistribution in a convenient manner for prostate cancer patients, opening the door for more…

How 3D printers can give robots a soft touch
Soft skin coverings and touch sensors have emerged as a promising feature for robots that are both safer and more intuitive for human interaction, but they are expensive and difficult…





















