Strong, Yet Gentle, Acid Uncovered
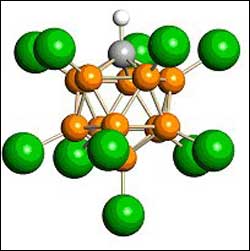
Carborane, part of the world’s strongest acid. [Atom color code: orange = boron, gray = carbon, green = chlorine, white = hydrogen.]
New Acid Has Potential to Help With a Variety of Processes
Researchers at the University of California, Riverside have discovered the world’s strongest acid. Remarkably it is also the gentlest acid. This non-toxic and non-corrosive acid may have a role in processes such as improving the quality of gasoline, developing polymers and synthesizing pharmaceuticals.
So how can an acid be both strong and gentle? The answer lies in the way chemists define the strength of an acid. Acid strength is the ability of an acid to add a hydrogen ion (H ) to basic molecules. On the other hand, corrosiveness has a lot to do with the nature of the negatively charged part of an acid that always accompanies H .
For example, hydrofluoric acid (chemical formula HF) dissolves glass because the fluoride (F) part of the acid attacks the silicon atom in silica glass at the same time that H attacks an oxygen atom, according to UC Riverside Distinguished Professor of Chemistry, Christopher Reed one of the authors of the findings.
Another example is the choice of an acid to clean lime deposits from inside a copper kettle, he pointed out. The wise homeowner chooses hydrochloric acid not nitric acid because the chlorine part of hydrochloric acid does not attack copper whereas the nitrate part of nitric acid would dissolve the kettle in a mess of toxic brown fumes.
The findings were published in the Oct. 11 issue of Angewandte Chemie in a paper titled “The Strongest Isolable Acid,” co-authored with Reed, UCR Colleagues Mark Juhasz, Stephan Hoffmann and Kee-Chan Kim, and Evgenii Stoyanov of the Boreskov Institute of Catalysis in Novosibirsk, Russia.
The new “strong-yet-gentle” acids are called carborane acids. The secret to their strength is twofold. Most importantly, the carborane part of the acid is an extremely weak base (i.e. weakly alkaline), weaker than the fluorosulfate part of fluorosulfuric acid, which was the previous record holder for the strongest acid. Secondly, carboranes have extraordinary chemical stability.
They have an icosahedral arrangement of eleven boron atoms plus one carbon atom, which is probably the most chemically stable cluster of atoms in all of chemistry, according to Reed. This means that the carborane part of the acid cannot participate in the chemistry of corrosion and decomposition that fluoride and nitrate show in hydrofluoric acid and nitric acid. As a result, carborane acids can add hydrogen ions to weakly basic molecules without destroying the often delicate positively charged molecules that are formed.This is the essence of their strong-yet-gentle qualities, Reed added.
Examples of molecules that add a hydrogen ion and are stabilized with a carborane as the negatively charged part of the product include benzene to give benzenium ion, C60 to give “protonated buckyball,” and alkenes to give carbocations.
None of these positively charged molecules had been “put in a bottle” at room temperature before because the acids used previously would decompose them. The strong-yet-gentle carborane acids overcome this difficulty, allowing chemists to take a closer look at important molecules whose existence was typically fleeting, Reed said. Acidified molecules are important short-lived intermediates in a huge variety of acid-catalyzed chemical transformations including the digestion of food, gasoline improvement, polymer formation and the synthesis of pharmaceuticals.
How strong are carborane acids? The strongest one is at least a million times stronger than concentrated sulfuric acid (H2SO4) and hundreds of times stronger than the previous record holder, fluorosulfuric acid (HFSO3). Concentrated sulfuric acid is already more than a billion times (1012) stronger than dilute swimming pool acid or the acid in one’s stomach. Acidic media having or exceeding the acidity of carborane acids had been achieved previously by adding antimony pentafluoride (SbF5) to fluorosulfuric acid but these mixtures are very corrosive and have other limitations.
Acids that are this strong are called superacids and they react with hydrocarbons from oil in a process called hydrocarbon cracking. This is an important process for raising the octane levels of gasoline. The new acids could become very important for understanding and improving this process, Reed said. The 1994 Nobel Prize in Chemistry was awarded to George Olah at USC for his pioneering studies in this field. Carborane acids have advanced this field even further.
There are many other molecules whose reactions with traditional acids are messy and therefore not very useful. Carborane acids deliver very clean acidity without ferocity. Thus, cleaner acid-catalysis of reactions important to the manufacture of pharmaceutical drugs and petroleum products should be possible. Also, there are atoms such as the element Xenon (symbol Xe), which have so far resisted reaction with acid. Reed and his research group want to add hydrogen ions to Xe atoms “because it’s never been done before.”
Reed says, “Our research is driven by making molecules that have never been made before. Carborane acids are allowing us to do this. That is the true value of this research. Science gets advanced, and at the same time, students are experiencing the thrill of discovery as they become scientists.”
Media Contact
More Information:
http://www.ucr.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…

Innovative microscopy demystifies metabolism of Alzheimer’s
Researchers at UC San Diego have deployed state-of-the art imaging techniques to discover the metabolism driving Alzheimer’s disease; results suggest new treatment strategies. Alzheimer’s disease causes significant problems with memory,…

A cause of immunodeficiency identified
After stroke and heart attack: Every year, between 250,000 and 300,000 people in Germany suffer from a stroke or heart attack. These patients suffer immune disturbances and are very frequently…





















