Spun from bone
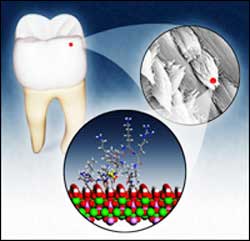
Sculpting Enamel From Bone: Top right: an electron microscope captures the weave structure of the long crystal stands that give enamel its strength. Bottom: A model based on nuclear magnetic resonance data derived at Pacific Northwest National Laboratory that shows how an active portion of the enamel-building protein, an amelogenin called LRAP, interacts with the crystal hydroxyapatite, or HAP, used by the body to engineer both bone and enamel. An isotopically labeled amino acid group (yellow, just left of center) in LRAP is near the HAP surface, a closeness that appears to encourage full interaction of protein with HAP that enables the protein to dictate the pattern of crystal formation.
PNNL-USC team discovers how protein in teeth controls bone-like crystals to form steely enamel
Bone and enamel start with the same calcium-phosphate crystal building material but end up quite different in structure and physical properties. The difference in bone and enamel microstructure is attributed to a key protein in enamel that molds crystals into strands thousands of times longer and much stronger than those in bone. The dimension of an enamel strand is 100,000 by 50 by 25 nanometers; bone is 35 by 25 by 4 nanometers.
But how that protein achieves this feat of crystal-strand shape-shifting has remained elusive. Today, scientists have reported the first direct observation of how this protein, amelogenin, interacts with crystals like those in bone to form the hard, protective enamel of teeth.
The study, published by a team from the Department of Energy’s Pacific Northwest National Laboratory and the University of Southern California on Friday (Sept. 24) in Journal of Biological Chemistry, identifies the region of the protein that interacts with the enamel crystals. The results explain how 100 nanometer spheres of amelogenin cluster like bowling balls around developing enamel crystals, forcing the crystals to elongate into thin, weaved strands that endow enamel with the strength of steel.
The discovery is a milestone for those who would wish to nano-engineer tissues, implants and synthetic coatings based on nature’s rules. “The proteins determine the crystal structure,” said Wendy J. Shaw, lead author and PNNL staff scientist. “Like bone, teeth are made of HAP, but the proteins present when teeth form create enamel, a material with entirely different properties from bone. If you can control the interactions between proteins and crystals, the same principal can be applied to nano-patterning and nano-building.”
Shaw’s co-authors are PNNL chief scientist Allison A. Campbell and Michael L. Paine and Malcolm L. Snead of USC’s Center for Craniofacial Molecular Biology in Los Angeles.
Earlier studies showed that mutated mice without amelogenin produced defective enamel. Other experiments set out to pinpoint the part of the protein responsible, pointing researchers toward the protein’s so-called carboxyl terminus—a region made up of many negatively charged amino acids. “People concentrated on this region,” according to Shaw, “because it has several negatively charged groups that are generally thought to interact with the positively charged groups in hydroxyapatite’’—or HAP, the crystals that make up bone and enamel.
A series of experiments confirmed that this region played an important role in shaping HAP crystals. Armed with this information, Shaw and colleagues set out to prove that this carboxyl group was indeed the business end of the protein.
To do that, they selected a form of amelogenin called LRAP and isotopically labeled one of the charged amino acids thought to be near LRAP’s surface. They put the protein into contact with hydroxyapatite, a proxy for developing enamel crystals, then took its picture. In this case, the “camera” was a powerful nuclear magnetic resonance instrument capable of recording the positions of tagged protein atoms in relation to the forming HAP crystals. “There are only a handful of labs capable of doing this,” Shaw said, “and there are more proteins than there are people to look at them all.”
The NMR data complement previous results, suggesting that protein’s function is to interact with HAP specifically. The carboxyl terminus of the protein is later cleaved by an enzyme, disrupting the protein-HAP interaction and allowing the long, thin crystals to grow outward as well, in three dimensions. The protein is cleaved further still, Shaw said, and by the time the process is complete, enamel is 99.9 percent crystal and no protein.
Media Contact
More Information:
http://www.pnl.govAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Bringing bio-inspired robots to life
Nebraska researcher Eric Markvicka gets NSF CAREER Award to pursue manufacture of novel materials for soft robotics and stretchable electronics. Engineers are increasingly eager to develop robots that mimic the…

Bella moths use poison to attract mates
Scientists are closer to finding out how. Pyrrolizidine alkaloids are as bitter and toxic as they are hard to pronounce. They’re produced by several different types of plants and are…

AI tool creates ‘synthetic’ images of cells
…for enhanced microscopy analysis. Observing individual cells through microscopes can reveal a range of important cell biological phenomena that frequently play a role in human diseases, but the process of…





















