A protein sequence associated with Huntington's disease may become life-saving vaccine component

The major component of every vaccine is an antigen that elicits specific immunity to a particular virus, bacteria or even cancer cells. Search for better antigens is one of the most pressing medical and public health necessities, and the biotech industry invests heavily in this research.
However, antigens are able to generate a limited immune response if administered alone. In order to be protective or therapeutic, vaccine compositions have to be supplemented with adjuvants, strong facilitators of the immune response. In the search for efficient adjuvants that are also non-toxic, scientists at Cure Lab, Inc. discovered a novel paradigm that capitalizes on insights from recent breakthroughs in understanding the fatal neurodegenerative disorder, Huntington's.
Huntington's disease results from mutations in the protein huntingtin, which contains a sequence of polyglutamine residues (polyQ). In healthy individuals, huntingtin contains less than 35 glutamines in the polyQ segment. However, in neurons of Huntington's patients, the number of glutamine residues in the sequence expands to more than 36. This induces protein self-aggregation, or formation of big protein “chunks”, and eventually causes neuronal dysfunction. Recent research findings indicate that attachment of a prolonged polyQ segment to almost any protein promotes its aggregation.
“There were two ideas – said Alex Shneider, Cure Lab's founder and CEO- which stimulated us to test if attachment of a long polyQ “tail” to an antigen can enhance immune response to vaccination”. First, he noticed that small aggregates formed by an antigen fused to a polyQ tail resembled the droplets that conventional adjuvants formed with antigenic peptides exposed on their surface. Dr. Shneider hypothesized that these polyQ containing aggregates were easily taken up by antigen-presenting cells, as is the case with adjuvant droplets, triggering the immune response.
Another hypothesis was proposed by Michael Sherman, professor at Boston University Medical School and scientific consultant at Cure Lab. “We knew- said Michael Sherman- that polyQ aggregates are destroyed in cells by a special degradation system, called autophagy. Interestingly, autophagy plays a key role in the processing of certain antigens, which is critical for the immune response. So, attachment of a polyQ tail should specifically target the antigen to autophagy, which in turn should facilitate the response. Thus, polyQ would serve as a molecular adjuvant”.
There are two branches to the immune system. One is responsible for developing antibodies against bacteria, viruses and toxins freely circulating in the organism. Another is aimed at eliminating virus-infected or cancer cells. Cure Lab's paper demonstrates that the new polyQ-based adjuvant efficiently enhances both branches of the immune response to a poor model antigen. Importantly, the adjuvant caused no apparent toxicity in mice.
Many questions remain to be answered. The adjuvant properties of the polyQ tail will have to be tested for each individual antigen of medical and veterinary importance. However, this work may launch a new era in vaccine development, the era of molecular adjuvants.
Media Contact
More Information:
http://www.curelab.comAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Wildfire danger to increase due to climate change
WSL Institute for Snow and Avalanche Research (SLF) researchers expect an elevated wildfire danger in the Alpine Foreland from 2040 onwards due to changing meteorological conditions. The danger currently remains…
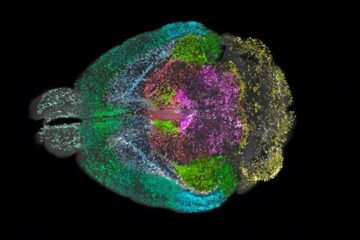
Advanced Brain Science Without Coding Expertise
Researchers at Helmholtz Munich and the LMU University Hospital Munich introduce DELiVR, offering a new AI-based approach to the complex task of brain cell mapping. The deep learning tool democratizes…

Transparent emissive microdisplays
… for ultra-light and compact augmented reality systems. As part of the HOT project (High-performance transparent and flexible microelectronics for photonic and optical applications), scientists from the Fraunhofer Institute for…





















