Mini-Donut Catches Chloride Ions

Ions—charged atoms or molecules—play an important role in nature, in our bodies as well as for science and technology. It is often necessary to trap, remove, mask, stabilize, or transport ions, whether in the body or the lab. With positively charged metal ions, this goal is often achieved with chelate ligands, organic molecules that tightly grab hold of the ions.
However, it is difficult to develop suitable chelators for negatively charged anions such as chloride and fluoride. Amar Flood and Yongjun Li at Indiana University (Bloomington, USA) have now synthesized a donut-shaped molecule that tightly and selectively takes chloride ions up into its center. As they report in the journal Angewandte Chemie , bridging hydrogen bonds are responsible for holding the chloride ion in place.
Chelators (from the Greek word for pincer) are small organic molecules that grab onto atoms or other small molecules, holding them by means of multiple binding sites. Chelate therapy is used to absorb and remove heavy metals in cases of poisoning, for example. It is a breeze to bind cations in this way. The development of organic molecules whose positively charged “arms” are arranged so as to tightly and selectively bind anions has not been successful to date.
Flood and Li found their new anion chelator more or less by coincidence when they were producing various macrocycles by means of an inexpensive, flexible synthetic technique known as “click chemistry”, which is a simple and efficient way to put molecules together into large entities. The researchers “clicked” four small rings together to form a large ring. This process also generates four more rings, made of three nitrogen atoms and two carbon atoms (triazole rings). These are not only by-products of the click chemistry, they are essential for binding the chloride ion, which can comfortably nestle into the empty center of the large donut-shaped ring. The triazoles hold on to the chloride ion by means of bridging hydrogen bonds, which is amazing because it was previously assumed that hydrogen bonds were not strong enough to form a sufficiently stable bond between a halogen ion and a chelate complex. It is probably vital that the binding sites in the structurally stable macrocycle are preorganized into the correct configuration so that the chelator does not have to rearrange itself around the ion before binding can occur, as is the case for open-chain chelators.
The four other nonbinding rings of the macrocycle can be varied almost as desired, so the researchers hope to generate a whole family of new chelators that are able to bind a spectrum of other anions with high specificity.
Author: Amar H. Flood, Indiana University, Bloomington (USA), http://flood.chem.indiana.edu/
Title: Pure C-H Hydrogen Bonding to Chloride Ions: A Preorganized and Rigid Macrocyclic Receptor
Angewandte Chemie International Edition 2008, 47, No. 14, 2649–2652, doi: 10.1002/anie.200704717
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Wildfire danger to increase due to climate change
WSL Institute for Snow and Avalanche Research (SLF) researchers expect an elevated wildfire danger in the Alpine Foreland from 2040 onwards due to changing meteorological conditions. The danger currently remains…
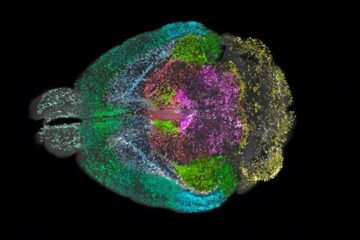
Advanced Brain Science Without Coding Expertise
Researchers at Helmholtz Munich and the LMU University Hospital Munich introduce DELiVR, offering a new AI-based approach to the complex task of brain cell mapping. The deep learning tool democratizes…
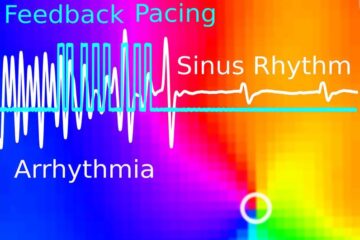
Gentle defibrillation for the heart
Using light pulses as a model for electrical defibrillation, Göttingen scientists developed a method to assess and modulate the heart function. The research team from the Max Planck Institute for…





















