Putting light-harvesters on the spot: how photosynthetic proteins get into the membrane
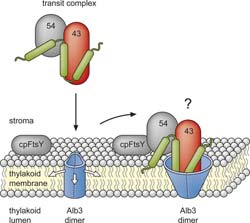
New transport model: Proteins of the light-harvesting complexes (green) have to be installed in special membranes inside the chloroplasts (thylakoid membranes). Soluble proteins (43, 54) transport them there. The membrane protein Alb3 forms a pore through interaction with one of the soluble proteins (43), through which the light-harvesting complex proteins are inserted into the membrane (Figure published in the Journal of Biological Chemistry) Figure: The American Society for Biochemistry and Molecular Biology<br>
How the light-harvesting complexes required for photosynthesis get to their site of action in the plant cell is reported by RUB biologists in the Journal of Biological Chemistry. The team led by Prof. Dr. Danja Schünemann (RUB working group on the molecular biology of plant organelles) has demonstrated for the first time that a membrane protein interacts with a single soluble protein to anchor the subunits of the light-harvesting complexes in the membrane. The researchers propose a new model that explains the integration into the membrane through the formation of a pore.
Light harvesting
Photosynthesis occurs in special areas of the plant cells, the chloroplasts, whereby the energy-converting process takes place in specific protein complexes (photosystems). To capture the light energy and efficiently transmit it to the photosystems, light-harvesting complexes are required which work like antenna. “The proteins of the light-harvesting complexes are the most abundant membrane proteins on Earth” says Dr. Beatrix Dünschede of the RUB. “There is a special transport mechanism that conveys them into the chloroplasts and incorporates them into the photosynthetic membrane”. Exactly how the various transport proteins interact with each other had, up to now, been unclear.
Interaction between only two proteins
Several soluble proteins and the membrane protein Alb3 that channels the proteins of the light-harvesting complexes into the membrane are involved in the transport. Bochum’s biologists examined intact, isolated plant cells and found that, for this purpose, Alb3 interacts with only a single soluble transport protein (cpSRP43). They confirmed this result in a second experiment with artificial membrane systems. “In a further experiment, we identified the region in Alb3 to which the soluble protein cpSRP43 binds” explains the RUB biologist Dr. Thomas Bals. “It turned out that the binding site is partly within the membrane and thus cannot be freely accessible for cpSRP43.”
Through the pore into the membrane
Schünemann’s team explains the data with a new model. The soluble transport proteins bind the proteins of the light-harvesting complexes and transport them to the membrane. There, the soluble transport protein cpSRP43 interacts with the membrane protein Alb3, which then forms a pore. The proteins of the light-harvesting complexes get into the pore, and from there they are released laterally into the membrane. “There are proteins in other organisms which are very similar to Alb3 and apparently also form pores” says Dünschede. “This supports our model. We are now planning new experiments in order to recreate the entire transport path in an artificial system.”
Bibliographic record
B. Dünschede, T. Bals, S. Funke, D. Schünemann (2011) Interaction studies between the chloroplast signal recognition particle subunit cpSRP43 and the full-length translocase Alb3 reveal a membrane-embedded binding region in Alb3, Journal of Biological Chemistry, 286, 35187-35195, doi: 10.1074/jbc.M111.250746
Further information
Working group on the molecular biology of plant organelles, Department for Biology and Biotechnology at the Ruhr-Universität, 44780 Bochum
Dr. Beatrix Dünschede, Tel. 0234/32-28467
beatrix.duenschede@rub.de
Dr. Thomas Bals, Tel. 0234/32-28467
thomas.bals@rub.de
Prof. Dr. Danja Schünemann, Tel: 0234/32-24293
danja.schuenemann@rub.de
Click for more
Homepage of the working group:
http://homepage.ruhr-uni-bochum.de/Danja.Schuenemann/Seiten_dt/index.html
Editor: Dr. Julia Weiler
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















