New property of flames sparks advances in technology

Published in the journal Angewandte Chemie, authors of the new study have discovered their previous understanding of how flames interact with a solid surface was mistaken. For the first time, they have demonstrated that a particular type of chemistry, called redox chemistry, can be accurately controlled at the surface.
This finding has wide implications for future technology, for example in detection of chemicals in the air, and in developing our understanding of the chemistry of lightning. It also opens up the possibility of being able to perform nitrogen oxide and carbon dioxide electrolysis at the source for the management of green house gases.
Results of the study show that depending on the chemical make-up of the flame, scientists can record a distinctive electrical fingerprint. The fingerprint is a consequence of the behaviour of specific chemical species at the surface of a solid conducting surface, where electrons can exchange at a very precise voltage.
Dr Daren Caruana, from the UCL Department of Chemistry, said: “Flames can be modelled to allow us to construct efficient burners and combustion engines. But the presence of charged species or ions and electrons in flames gives them a unique electrical property.”
Dr Caruana added: “By considering the gaseous flame plasma as an electrolyte, we show that it is possible to control redox reactions at the solid/gas interface.”
The team developed an electrode system which can be used to probe the chemical make-up of flames. By adding chemical species to the flame they were able to pick up current signals at specific voltages giving a unique electrochemical finger print, called a voltammogram.
The voltammograms for three different metal oxides – tungsten oxide, molybdenum oxide and vanadium oxide – are all unique. Furthermore, the team also demonstrated that the size of the current signatures depend on the amount of the oxide in the flame. Whilst this is possible and routinely done in liquids, this is the first time to be shown in the gas phase.
UCL chemists have shown that there are significant differences between solid/gas reactions and their liquid phase equivalents. Liquid free electrochemistry presents access to a vast number of redox reactions, current voltage signatures that lie outside potential limits defined by the liquid.
The prospect of new redox chemistries will enable new technological applications such as electrodeposition, electroanalysis and electrolysis, which will have significant economic and environmental benefits.
Dr Caruana said: “The mystique surrounding the properties of fire has always captivated our imagination. However, there are still some very significant technical and scientific questions that remain regarding fire and flame. “
1. For more information or to interview Dr Daren Caruana please contact Clare Ryan in the UCL Media Relations Office on tel: +44 (0)20 3108 3846, mobile: +44 07747 565 056, out of hours +44 (0)7917 271 364, e-mail: clare.ryan@ucl.ac.uk.
2. 'Dynamic electrochemistry in flame plasma electrolyte' is published online in the journal Angewandte Chemie. Copies of the paper are available from UCL Media Relations.
About UCL (University College London)
Founded in 1826, UCL was the first English university established after Oxford and Cambridge, the first to admit students regardless of race, class, religion or gender, and the first to provide systematic teaching of law, architecture and medicine. We are among the world's top universities, as reflected by performance in a range of international rankings and tables. UCL currently has 24,000 students from almost 140 countries, and more than 9,500 employees. Our annual income is over £800 million.
www.ucl.ac.uk | Follow us on Twitter @uclnews
Media Contact
More Information:
http://www.ucl.ac.ukAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles
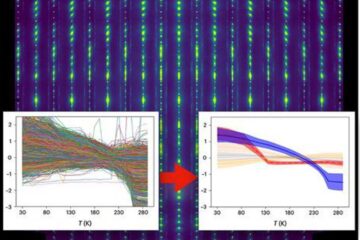
Machine learning algorithm reveals long-theorized glass phase in crystal
Scientists have found evidence of an elusive, glassy phase of matter that emerges when a crystal’s perfect internal pattern is disrupted. X-ray technology and machine learning converge to shed light…
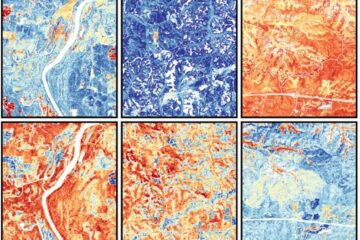
Mapping plant functional diversity from space
HKU ecologists revolutionize ecosystem monitoring with novel field-satellite integration. An international team of researchers, led by Professor Jin WU from the School of Biological Sciences at The University of Hong…

Inverters with constant full load capability
…enable an increase in the performance of electric drives. Overheating components significantly limit the performance of drivetrains in electric vehicles. Inverters in particular are subject to a high thermal load,…





















