Pressure Relief Valve in Cellular Membrane Identified
The volume-regulated anion channel VRAC (Scheme: Lab. Jentsch/Copyright: MDC/FMP)
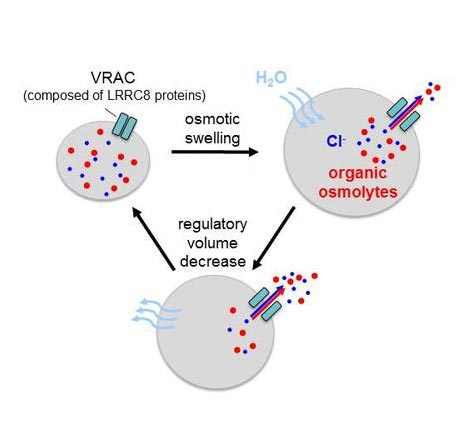
A certain chloride channel, a membrane protein that allows the passage of the chloride ion, is of crucial importance in volume regulation. It is activated by the swelling of the cell and then releases chloride ions and organic matter (osmolytes) from the cell. Researchers in Berlin-Buch have now succeeded for the first time in elucidating the molecular identity of this volume-regulated anion channel (VRAC) (Science Express, DOI: 10.1126/science.1252826)*.
Researchers led by Professor Thomas J. Jentsch (Max Delbrück Center for Molecular Medicine, MDC, Berlin-Buch/Leibniz-Institut für Molekulare Pharmakologie, FMP) identified a molecule, LRRC8A, which is an essential constituent of the volume-regulated anion channel (VRAC). This protein needs to be assembled with related proteins (LRRC8B to E) to form channels with probably six subunits.
They could also show for the first time that these chloride channels are also permeable to small organic molecules such as taurine or amino acids. For over 20 years, research groups across the globe have been seeking to elucidate the molecular structure of the volume-regulated anion channel (VRAC). It took Jentsch’s team almost four years to achieve this breakthrough.
The regulation of cell volume is important for many functions in the organism. The volume-regulated anion channel (VRAC) which Thomas Jentsch and his coworkers Felizia Voss and Tobias Stauber now identified at the molecular level is expressed in all vertebrate cells.
If a particular cell volume is exceeded, the channel opens and permits the outflow of osmolytes such as chloride ions as well as small organic molecules such as taurine and amino acids. By contrast, cations such as potassium or sodium cannot permeate.
Once the channel is opened, chloride and other osmolytes pass in a passive process called diffusion. Due to its biophysical properties the channel only allows anions and certain organic compounds to pass. Thus, the cell reduces the concentration of its osmolytically active constituents to (or even below) that of the surrounding fluid. At the same time, the water content of the cell decreases as the water molecules flow out via aquaporins in the cell membrane. The volume of the cell decreases again.
LRRC8A was discovered as a VRAC component using a genome-wide RNA interference (siRNA) screen in collaboration with Katina Lazarow and Jens von Kries from the FMP Screening Unit. By means of short RNA snippets, the translation of the genetic information into the corresponding proteins can be suppressed. Using a one-by-one approach in a large-scale cell culture experiment, the Berlin group transiently silenced the products of all approximately 20,000 human genes.
In an automated screening process the researchers investigated which of the genes are required for the swelling-activated anion flux across the cell membrane. The approximately 130,000 time-dependent ion flux measurements were statistically analyzed with help from the Bioinformatics Group of the MDC (Nancy Mah/Miguel Andrade-Navarro).
The essential role of LRRC8 proteins in the volume-regulated anion channel was verified using CRISPR/Cas technology, which just became available during the past two years. With this method, specific genes on the chromosomes can be disrupted completely. Different combinations of LRRC8 proteins, all including the obligate LRRC8A, – either by omitting some of the family members from gene disruption or by reconstituting different combinations – led to different electrophysiological properties of the channel. “This allows us to explain the behavior of the channel in different tissues which until now had remained elusive,” Thomas Jentsch said.
“Cells can swell or in the worst case even burst. Water transport and content must therefore be tightly regulated,” he added. Water transport is always driven by the osmotic gradient. Cells take up chloride from their surroundings, whereas organic substances such as taurine or amino acids are produced within the cells.
Deciphering the molecular structure of this chloride channel may also pave the way for better medical treatments, for example, after stroke. “In the case of damage in the brain, cells swell and release glutamate, which acts upon receptors on nerve cells. The subsequent inflow of calcium raises the intracellular concentration of this ion to toxic levels,” Jentsch said. With the onset of programmed cell death (apoptosis) during cancer chemotherapy, however, there is a strong reduction in cell volume. The volume-regulated chloride channel also appears to be involved in this process.
*Identification of LRRC8 Heteromers as Essential Component of the Volume-regulated Anion Channel VRAC.
Felizia K. Voss1,2,3, Florian Ullrich1,2,3, Jonas Münch1,2,3, Katina Lazarow1, Darius Lutter1,2,3, Nancy Mah2, Miguel A. Andrade-Navarro2, Jens P. von Kries1, Tobias Stauber1,2 * and Thomas J. Jentsch1,2,4 *
*Correspondence to: Jentsch@fmp-berlin.de (T.J.J.); tstauber@fmp-berlin.de (T.S.).
1Leibniz-Institut für Molekulare Pharmakologie (FMP), Berlin
2Max Delbrück Center for Molecular Medicine (MDC), Berlin
3Graduate program of the Freie Universität Berlin
4Neurocure, Charité Universitätsmedizin, Berlin
Science Express, 10. April 2014; DOI: 10.1126/science.1252826
Contact:
Barbara Bachtler
Press Department
Max Delbrück Center for Molecular Medicine (MDC) Berlin-Buch
in the Helmholtz Association
Robert-Rössle-Straße 10
13125 Berlin
Germany
Phone: +49 (0) 30 94 06 – 38 96
Fax: +49 (0) 30 94 06 – 38 33
e-mail: presse@mdc-berlin.de
http://www.mdc-berlin.de/
Silke Oßwald
Public Relations
Leibniz-Institut für Molekulare Pharmakologie
im Forschungsverbund Berlin e.V. (FMP)
Campus Berlin-Buch
Robert-Roessle-Str. 10
13125 Berlin, Germany
Phone: +49-30-94793-104
e-mail: osswald@fmp-berlin.de
http://www.fmp-berlin.info/de/home.html
The Max Delbrück Center for Molecular Medicine (MDC) is one of 18 research centers of the Helmholtz Association. It was founded in 1992 to link basic molecular basic research with clinical research. The MDC is working closely with the Charité – University Medicine in the Berlin Institute of Health (BIH) and has evolved in recent years into an internationally recognized research institute.
The Leibniz-Institut für Molekulare Pharmakologie (FMP) is part of the Forschungsverbund Berlin e.V. (FVB), a federation of eight institutes in Berlin in the field of natural, life and environmental sciences with a staff of more than 1500 employees. The multiple award-winning institutions are members of the Leibniz Association. The Forschungsverbund came into being in 1992 in a unique historical situation as the successor organization of the former Academy of Sciences of the GDR.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















