Patient stem cells used to make 'heart disease-on-a-chip'
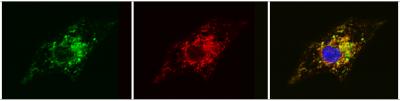
Researchers use modified RNA transfection to correct genetic dysfunction in heart stem cells derived from Barth syndrome patients. The series of images show how inserting modified RNA into diseased cells causes the cells to produce functioning versions of the TAZ protein (first image: in green) that correctly localize in the mitochondria (second image: in red). When the images are merged to demonstrate this localization, green overlaps with red, giving the third image a yellow color. Credit: Gang Wang and William Pu/Boston Children's Hospital
Harvard scientists have merged stem cell and 'organ-on-a-chip' technologies to grow, for the first time, functioning human heart tissue carrying an inherited cardiovascular disease. The research appears to be a big step forward for personalized medicine, as it is working proof that a chunk of tissue containing a patient's specific genetic disorder can be replicated in the laboratory.
The work, published in Nature Medicine, is the result of a collaborative effort bringing together scientists from the Harvard Stem Cell Institute, the Wyss Institute for Biologically Inspired Engineering, Boston Children's Hospital, the Harvard School of Engineering and Applied Sciences, and Harvard Medical School. It combines the 'organs-on-chips' expertise of Kevin Kit Parker, PhD, and stem cell and clinical insights by William Pu, MD.
Using their interdisciplinary approach, the investigators modeled the cardiovascular disease Barth syndrome, a rare X-linked cardiac disorder caused by mutation of a single gene called Tafazzin, or TAZ. The disorder, which is currently untreatable, primarily appears in boys, and is associated with a number of symptoms affecting heart and skeletal muscle function.
The researchers took skin cells from two Barth syndrome patients, and manipulated the cells to become stem cells that carried these patients' TAZ mutations. Instead of using the stem cells to generate single heart cells in a dish, the cells were grown on chips lined with human extracellular matrix proteins that mimic their natural environment, tricking the cells into joining together as they would if they were forming a diseased human heart. The engineered diseased tissue contracted very weakly, as would the heart muscle seen in Barth syndrome patients.
The investigators then used genome editing—a technique pioneered by Harvard collaborator George Church, PhD—to mutate TAZ in normal cells, confirming that this mutation is sufficient to cause weak contraction in the engineered tissue. On the other hand, delivering the TAZ gene product to diseased tissue in the laboratory corrected the contractile defect, creating the first tissue-based model of correction of a genetic heart disease.
“You don't really understand the meaning of a single cell's genetic mutation until you build a huge chunk of organ and see how it functions or doesn't function,” said Parker, who has spent over a decade working on 'organs-on-chips' technology. “In the case of the cells grown out of patients with Barth syndrome, we saw much weaker contractions and irregular tissue assembly. Being able to model the disease from a single cell all the way up to heart tissue, I think that's a big advance.”
Furthermore, the scientists discovered that the TAZ mutation works in such a way to disrupt the normal activity of mitochondria, often called the power plants of the cell for their role in making energy. However, the mutation didn't seem to affect overall energy supply of the cells. In what could be a newly identified function for mitochondria, the researchers describe a direct link between mitochondrial function and a heart cell's ability to build itself in a way that allows it to contract.
“The TAZ mutation makes Barth syndrome cells produce an excess amount of reactive oxygen species or ROS—a normal byproduct of cellular metabolism released by mitochondria—which had not been recognized as an important part of this disease,” said Pu, who cares for patients with the disorder.
“We showed that, at least in the laboratory, if you quench the excessive ROS production then you can restore contractile function,” Pu added. “Now, whether that can be achieved in an animal model or a patient is a different story, but if that could be done, it would suggest a new therapeutic angle.”
His team is now trying to translate this finding by doing ROS therapy and gene replacement therapy in animal models of Barth syndrome to see if anything could potentially help human patients. At the same time, the scientists are using their human 'heart disease-on-a-chip' as a testing platform for drugs that are potentially under trial or already approved that might be useful to treat the disorder.
“We tried to thread multiple needles at once and it certainly paid off,” Parker said. “I feel that the technology that we've got arms industry and university-based researchers with the tools they need to go after this disease.”
Both Parker and Pu, who first talked about collaborating at a 2012 Stockholm conference, credit their partnership and scientific consilience for the success of this research. Parker asserted that the 'organs-on-chips' technology that has been a flagship of his lab only worked so fast and well because of the high quality of Pu's patient-derived cardiac cells.
“When we first got those cells down on the chip, Megan, one of the joint first authors, texted me 'this is working,'” he recalled. “We thought we'd have a much harder fight.”
“When I'm asked what's unique about being at Harvard, I always bring up this story,” Pu said. “The diverse set of people and cutting-edge technology available at Harvard certainly made this study possible.”
The researchers also involved in this work include: Joint first authors Gang Wang, MD, of Boston Children's Hospital, and Megan McCain, PhD, who earned her degree at the Harvard School of Engineering and Applied Sciences and is now an assistant professor at the University of Southern California. Amy Roberts, MD, of Boston Children's Hospital, and Richard Kelley, MD, PhD, at the Kennedy Krieger Institute provided patient data and samples, and Frédéric Vaz, PhD, and his team at the Academic Medical Center in the Netherlands conducted additional analyses. Technical protocols were shared by Kenneth Chien, MD, PhD, at the Karolinska Institutet.
Kevin Kit Parker, PhD, is the Tarr Family Professor of Bioengineering and Applied Physics in Harvard's School of Engineering and Applied Sciences, a Core Faculty member of the Wyss Institute for Biologically Inspired Engineering, and a Principal Faculty member of the Harvard Stem Cell Institute.
William Pu, MD, is an Associate Professor at Harvard Medical School, a member of the Department of Cardiology at Boston Children's Hospital, and an Affiliated Faculty member of the Harvard Stem Cell Institute.
George Church, PhD, is a Professor of Genetics at Harvard Medical School and a Core Faculty member of the Wyss Institute of Biologically Inspired Engineering.
The work was supported by the Barth Syndrome Foundation, Boston Children's Hospital, the National Institutes of Health, and charitable donations from Edward Marram, Karen Carpenter, and Gail Federici Smith.
Cited: Wang, G., McCain, M., et. al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with iPSC and heart-on-a-chip technologies. Nature Medicine. May 11, 2014
Media Contact
More Information:
http://www.harvard.edu/All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















