Oversized fat droplets: Too much of a good thing
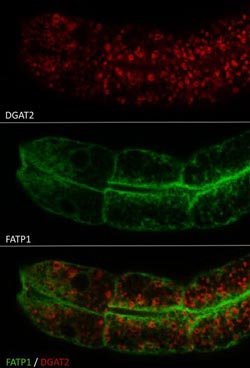
DGAT2 (shown in red) and FATP1 (shown in green) regulate the size of lipid droplets to accommodate increased amounts of intracellular fat.<br><br>Credit: Dr. Ningyi Xu, Stowers Institute for Medical Research<br>
As the national waistline expands, so do pools of intra-cellular fat known as lipid droplets. Although most of us wish our lipid droplets would vanish, they represent a cellular paradox: on the one hand droplets play beneficial roles by corralling fat into non-toxic organelles. On the other, oversized lipid droplets are associated with obesity and its associated health hazards.
Until recently researchers understood little about factors that regulate lipid droplet size. Now, a study from the Stowers Institute of Medical Research published in an upcoming issue of Journal of Cell Biology reports a genetic screen of roundworms that identifies two proteins required for the dramatic expansion of lipid droplets. That study, from the lab of Assistant Investigator Ho Yi Mak, Ph.D., sheds new light onto the molecular processes linked to fat metabolism.
“In worms and mammals lipid droplets are evolutionarily conserved structures that store cellular fat as triglycerides, a benign form of fat,” says Mak, whose lab relies heavily on the roundworm C. elegans to evaluate the genetics and biochemistry of fat storage. “Currently, there is a great appreciation that a diverse range of systems can be exploited to understand where triglycerides are synthesized and how they get stored in lipid droplets.”
Prior to the Journal of Cell Biology study, Mak's lab and others had shown that some enzymes that direct triglyceride synthesis are physically located in a network of intracellular tubules called the endoplasmic reticulum (ER), suggesting that the ER communicates with lipid droplets. In fact, high magnification imaging of single cells showed that ER membranes often “push up” against droplets, suggesting they might in some way load them.
To determine if this was the case, the Mak team employed mutant roundworms they previously discovered that displayed abnormally large-sized lipid droplets. Using genetic techniques, Mak introduced additional mutations in the genome of these worms to search for hits that restored droplets to normal size.
That effort revealed that disruption of two genes that encode proteins named FATP1 and DGAT2 did just that: hits in either shrank fat droplets to normal size. Further biochemical analysis showed that FATP1 and DGAT2, which catalyze sequential steps in triglyceride synthesis, were closely associated in a protein complex, strongly suggesting that they act in a two-step process required to form out-sized droplets in the first place.
Most interestingly, both FATP1 and DGAT2 resided in the right cellular space: imaging of living worms revealed that FATP1 resides in ER membranes, while DGAT2 is enriched at the surface of the droplets, suggesting an anatomical link between the two enzymes regulating triglyceride biosynthesis.
Finally, the team demonstrated the relevance of this mechanism to mammalian cells by expressing mouse versions of FATP1 and DGAT2 in cultured cells. They then added a fatty acid building block of triglycerides to the culture media—the equivalent of feeding cells a pizza—and monitored fat storage.
“What we saw was that again the two proteins were in close proximity to each other in cells and acted synergistically to allow cells to store more fat and expand the size of lipid droplets,” says Mak. “This shows that coupling of FATP1 and DGAT2 seen in worms is evolutionarily conserved in mammalian cells.”
Mak cautions that no one should assume that simply inactivating FATP1 and DGAT2 could be a panacea to weight gain: apparently, there is one thing worse than having an oversized fat droplet, and that's not being able to form one at all. “One side of this issue suggests that lipid droplet formation is actually protective,” he says, noting that failure to sequester fat into droplets can cause cellular stress and insulin resistance. “By the time you see large lipid droplets, tissues are trying very hard to contain the harmful effects of excess fat.”
Thus, although out-sized lipid droplets are often observed in liver and muscle cells of obese individuals, the toxic conditions that trigger obesity-related conditions like diabetes may emerge when fat depots can no longer expand.
On the flip side, Mak notes that endurance athletes also display oversized lipid droplets in muscle cells. “Lipid droplets store a rich form of energy,” he says. “Having a high energy depot on site likely allows muscle tissues use them as a sustained form of energy.”
Mak's team will now address whether nutrient intake regulates FATP1-DGAT2 activity and hence droplet size. “Right now the worm is our primary discovery tool,” he says. “But we will continue to extend our studies to mammalian cell culture models. We want to know whether the FATP1-DGAT2 complex becomes more active after you eat a Big Mac or a piece of apple pie.”
The study's first author was Ningyi Xu, a postdoctoral fellow in the Mak lab. Also contributing to the study were Shaobing O. Zhang, Ronald A. Cole, Sean A. McKinney, Fengli Guo, and Sudheer Bobba—all of the Stowers Institute—and Robert V. Farese, Jr., and Joel T. Haas, both of the University of California, San Francisco.
The work was supported by funding from the Stowers Institute for Medical Research and the National Institutes of Health.
About the Stowers Institute for Medical Research
The Stowers Institute for Medical Research is a non-profit, basic biomedical research organization dedicated to improving human health by studying the fundamental processes of life. Jim Stowers, founder of American Century Investments, and his wife, Virginia, opened the Institute in 2000. Since then, the Institute has spent over 900 million dollars in pursuit of its mission.
Currently, the Institute is home to nearly 550 researchers and support personnel; over 20 independent research programs; and more than a dozen technology-development and core facilities.
Media Contact
More Information:
http://www.stowers.orgAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















