News from the Mailroom of the Cell – Max Planck Researchers elucidate mechanism of protein
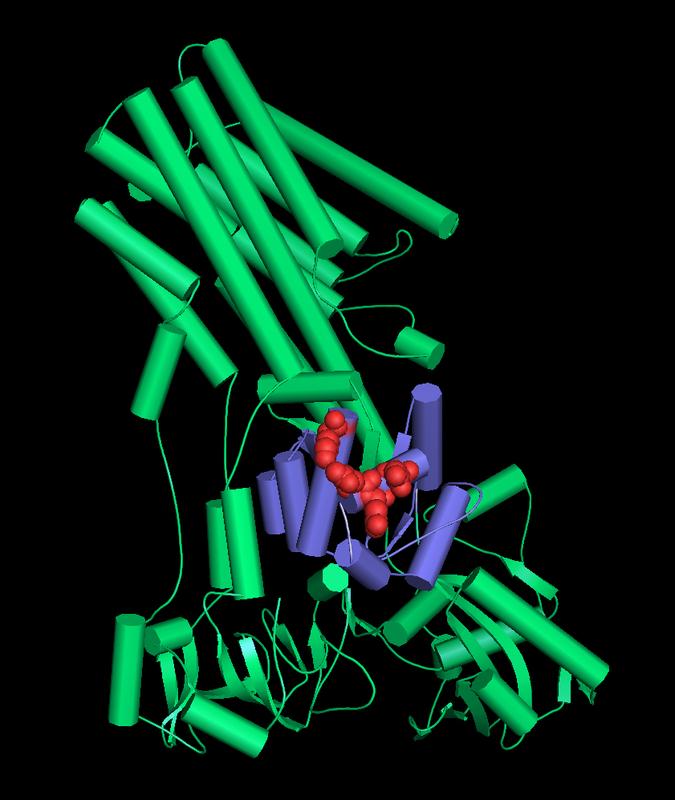
Structural model of the human calcium transporter SPCA1: The cofilin binding domain is displayed in purple. The four residues required for direct cofilin binding are indicated as red spheres. Figure: Dr. Bianca Habermann / Copyright: MPI of Biochemistry
Researchers at the MPI of Biochemistry (MPIB) have now shown in detail how the ‘mailroom of the cell’ sorts a subset of the proteins that are transported out of the cell. “This mechanism regulates the correct sorting of the proteins and is thus essential for the human organism,” said Julia von Blume, research group leader at the MPIB. The results have been published in the Journal of Cell Biology.
Similarly as we people communicate, our body cells can also exchange brief messages. For this purpose, many of them often use proteins as messengers, such as hormones. These are produced inside the cell and then packed for export in small membrane packages, the vesicles, and sent out from a kind of ‘mailroom’, the so-called Golgi apparatus. But since not all vesicles are destined for export, it is crucial that the proteins are sorted into the correct vesicles. Until now, however, it was unclear how this sorting actually works.
Julia von Blume and her team of the research group “Molecular Basis of Protein Trafficking” at the MPIB are dedicated to finding the answer to this question. In previous studies they showed that the interaction of three proteins is decisive for the sorting process. Actin, a central support and transport molecule, and cofilin, a protein complex, work together. Near the vesicles they bind the calcium transporter SPCA1, which increases the local concentration of calcium. That attracts the corresponding proteins, which are then incorporated into a vesicle and exported from the cell.
In the current publication, the scientists elucidated the specific molecular mechanism underlying these steps. By investigating the individual proteins in isolation as well as in living cells, they were able to precisely decipher their interaction (down to the amino acids).
The new findings not only confirm the previous studies, but are also medically relevant: “This mechanism regulates the correct sorting of the proteins in the Golgi apparatus and is thus essential for the human organism,” said Julia von Blume, illustrating her explanation with an example: “If this process is impaired, severe health problems such as the skin disease Hailey-Hailey can be the result. It is assumed that due to a genetic defect, the calcium transporter SPCA1 does not function and therefore certain proteins that are important for cell-cell communication in the epidermis can no longer be exported from the cell.“ Patients with Hailey-Hailey disease thus suffer from discoloration of the skin, itching and blisters.
In the future, the scientists hope to clarify whether other proteins are involved in the process – always with the objective to ultimately reconstruct the entire process.
[HS]
Original publication:
C. Kienzle, N. Basnet, A. Crevenna, G. Beck, B. Habermann, N. Mizuno and J. von Blume: Cofilin recruits F-actin to SPCA1 and promotes Ca2+-mediated secretory cargo sorting. Journal of Cell Biology, September 1, 2014.
DOI: 10.1083/jcb.201311052
Contact:
Dr. Julia von Blume
Molecular Basis of Protein Trafficking
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
Germany
e-mail: vonblume@biochem.mpg.de
http://www.biochem.mpg.de/blume
Anja Konschak
Public Relations
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
Germany
Phone: +49 89 8578-2824
e-mail: konschak@biochem.mpg.de
http://www.biochem.mpg.de
http://www.biochem.mpg.de/blume
http://Website of the Research Group “Molecular Basis of Protein Trafficking” (Julia von Blume)
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Machine learning algorithm reveals long-theorized glass phase in crystal
Scientists have found evidence of an elusive, glassy phase of matter that emerges when a crystal’s perfect internal pattern is disrupted. X-ray technology and machine learning converge to shed light…

Mapping plant functional diversity from space
HKU ecologists revolutionize ecosystem monitoring with novel field-satellite integration. An international team of researchers, led by Professor Jin WU from the School of Biological Sciences at The University of Hong…

Inverters with constant full load capability
…enable an increase in the performance of electric drives. Overheating components significantly limit the performance of drivetrains in electric vehicles. Inverters in particular are subject to a high thermal load,…





















