Myriad myPath melanoma test improves the reliability of melanoma diagnosis

Myriad Genetics, Inc. (Nasdaq: MYGN) today presented results from a pivotal clinical validation study of the Myriad myPath™ Melanoma test at the 2014 American Society of Clinical Oncology (ASCO) annual meeting in Chicago, Ill. The Myriad myPath Melanoma test is a novel molecular test that accurately differentiates malignant melanoma from benign skin lesions with a high level of accuracy and helps physicians deliver a more objective and confident diagnosis for patients.
“Unfortunately, some melanomas mimic benign skin lesions, making them very difficult to diagnose and an uncertain result is confusing for patients and clinicians. What we need is a new tool to help us make a more definitive diagnosis,” said Sancy Leachman, M.D., Ph.D, chair of the Department of Dermatology at the Oregon Health & Science University (OHSU) School of Medicine and director of the Melanoma Research Program at the Knight Cancer Institute. “In the validation study, Myriad myPath Melanoma was shown to differentiate malignant melanoma from benign skin lesions using traditional dermatopathology as a gold standard. This represents a significant contribution toward making a prompt and accurate diagnosis of potentially fatal melanoma.”
The clinical validation study evaluated 437 pigmented lesions (211 melanomas and 226 nevi) representing a broad spectrum of subtypes submitted from four academic medical centers in the United States. The clinical endpoint was the concordance of the Myriad myPath Melanoma test to a consensus diagnosis from expert dermatopathologists. In this study, the Myriad myPath Melanoma test effectively differentiated malignant melanoma from benign skin lesions with a sensitivity of 90 percent and a specificity of 91 percent. These results strongly support the clinical use of the Myriad myPath Melanoma test as an adjunct to standard pathology techniques in the evaluation of pigmented skin lesions, particularly in difficult-to-classify cases.
The Myriad myPath Melanoma test has now shown reproducible results in two large cohorts. Last November, Myriad presented results from its verification study at the American Society of Dermatopathology annual meeting. Data from that verification study of 464 lesions showed that the Myriad myPath Melanoma test had greater than a 90 percent diagnostic accuracy in differentiating malignant melanoma from benign skin lesions in a variety of subtypes.
Additionally, an analysis of a prospective clinical utility study was highlighted at ASCO and initial findings from that study are consistent with earlier findings from a retrospective clinical utility study that was presented at the United States & Canadian Academy of Pathology's (USCAP) annual meeting in March. The USCAP data demonstrated a 33 percent change in medical management based upon the Myriad myPath Melanoma test result.
“We believe the Myriad myPath Melanoma test will substantially improve the standard of care for patients with melanoma,” said Loren Clarke, M.D., vice president of Medical Affairs at Myriad Genetic Laboratories. “The Myriad myPath Melanoma test objectively answers a vital clinical question for healthcare providers: Does my patient have malignant melanoma that requires surgical or medical intervention, or a harmless skin lesion that only needs to be watched? The appropriate therapy may differ drastically depending on the answer to that question.”
About Myriad myPath Melanoma Testing
The Myriad myPath Melanoma test is a clinically validated gene expression test designed to differentiate malignant melanoma from benign nevi across all major melanoma subtypes. The Myriad myPath Melanoma test is a unique test of 23 genes that provides valuable, additive diagnostic information unavailable from any other method – information that can help physicians deliver a more confident diagnosis.
Melanoma is the most serious type of skin cancer. According to the American Cancer Society, about 76,000 new melanomas are diagnosed each year and more than 9,000 people die from the disease annually. Each year in the United States, there are approximately 1.5 million skin biopsies performed specifically for the diagnosis of melanoma, and approximately 14 percent or 210,000 biopsies are classified as indeterminate, meaning that the dermatopathologist cannot confidently determine whether the cells are benign or malignant. For more information visit: http://www.isthismelanoma.com and http://www.myriadpro.com/melanoma.
About Myriad Genetics
Myriad Genetics is a leading molecular diagnostic company dedicated to making a difference in patients' lives through the discovery and commercialization of transformative tests to assess a person's risk of developing disease, guide treatment decisions and assess risk of disease progression and recurrence. Myriad's molecular diagnostic tests are based on an understanding of the role genes play in human disease and were developed with a commitment to improving an individual's decision making process for monitoring and treating disease. Myriad is focused on strategic directives to introduce new products, including companion diagnostics, as well as expanding internationally. For more information on how Myriad is making a difference, please visit the Company's website: http://www.myriad.com. Myriad, the Myriad logo, Prolaris, Myriad myPath, Myriad myPlan and Myriad myRisk are trademarks or registered trademarks of Myriad Genetics, Inc. in the United States and foreign countries. MYGN-F, MYGN-G.
Safe Harbor Statement
This press release contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including statements relating to the presentation of the Myriad myPath Melanoma test clinical study data at the 2014 ASCO Annual Meeting; the effectiveness of Myriad myPath testing to accurately differentiate malignant melanoma from benign lesions and help physicians deliver a more objective and confident diagnosis; the Company's belief that the Myriad myPath Melanoma test represents a significant advancement in the prompt and accurate diagnosis of potentially fatal melanoma; the clinical use of the Myriad myPath Melanoma test as an adjunct to standard pathology techniques in the evaluation of pigmented skin lesions, particularly in difficult-to-classify cases; the Company's belief that the Myriad myPath Melanoma test will substantially improve the standard of care for patients with melanoma; and the Company's strategic directives under the captions “About Myriad myPath Melanoma Testing” and “About Myriad Genetics.” These “forward-looking statements” are management's present expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to: the risk that sales and profit margins of our existing molecular diagnostic tests and companion diagnostic services may decline or will not continue to increase at historical rates; risks related to changes in the governmental or private insurers reimbursement levels for our tests; the risk that we may be unable to develop or achieve commercial success for additional molecular diagnostic tests and companion diagnostic services in a timely manner, or at all; the risk that we may not successfully develop new markets for our molecular diagnostic tests and companion diagnostic services, including our ability to successfully generate revenue outside the United States; the risk that licenses to the technology underlying our molecular diagnostic tests and companion diagnostic services tests and any future tests are terminated or cannot be maintained on satisfactory terms; risks related to delays or other problems with operating our laboratory testing facilities; risks related to public concern over our genetic testing in general or our tests in particular; risks related to regulatory requirements or enforcement in the United States and foreign countries and changes in the structure of the healthcare system or healthcare payment systems; risks related to our ability to obtain new corporate collaborations or licenses and acquire new technologies or businesses on satisfactory terms, if at all; risks related to our ability to successfully integrate and derive benefits from any technologies or businesses that we license or acquire; risks related to increased competition and the development of new competing tests and services; the risk that we or our licensors may be unable to protect or that third parties will infringe the proprietary technologies underlying our tests; the risk of patent-infringement claims or challenges to the validity of our patents; risks related to changes in intellectual property laws covering our molecular diagnostic tests and companion diagnostic services and patents or enforcement in the United States and foreign countries, such as the Supreme Court decision in the lawsuit brought against us by the Association for Molecular Pathology et al; risks of new, changing and competitive technologies and regulations in the United States and internationally; and other factors discussed under the heading “Risk Factors” contained in Item 1A of our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission, as well as any updates to those risk factors filed from time to time in our Quarterly Reports on Form 10-Q or Current Reports on Form 8-K. All information in this press release is as of the date of the release, and Myriad undertakes no duty to update this information unless required by law.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles
A chip unique in the world
A team from UPV and iPRONICS has manufactured the first universal, programmable and multifunctional photonic chip on the market. A team from the Photonics Research Laboratory (PRL)-iTEAM of the Universitat…

Advance in light-based computing
…shows capabilities for future smart cameras. UCLA-developed experimental device demonstrates ability to reduce glare in images. Researchers developing the next generation of computing technology aim to bring some light to…
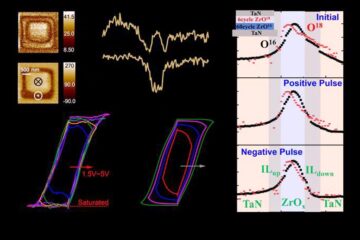
Evidence for reversible oxygen ion movement during electrical pulsing
…enabler of the emerging ferroelectricity in binary oxides. In a recent study published in Materials Futures, researchers have uncovered a pivotal mechanism driving the emergence of ferroelectricity in binary oxides….





















