Molecular Hinges Open Pathways
Modern technology makes extensive use of ion exchangers. For example, they are commonly used to decalcify water by binding calcium ions and releasing sodium ions in return. Good exchangers tend to be materials with high surface areas, such as resins, zeolites, or clays. German scientists have now demonstrated that the compact, crystalline structures of intermetallic compounds, in which the diffusion pathways for efficient materials transport are actually absent, can also exchange ions. In the journal Angewandte Chemie, they report the full replacement of the chloride ions in Bi12Rh3Cl2 crystals by bismuth atoms.
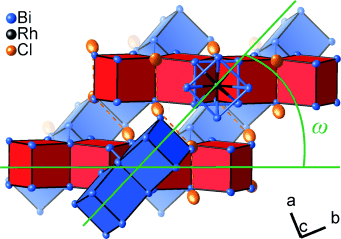
The team working with Michael Ruck at the Technical University of Dresden noticed this unexpected phenomenon while researching bismuth subhalides. Subhalides are compounds that have fewer halogen ions than pure ionic metal halides. This results in regions that contain direct bonds between metal atoms. Subhalides with bismuth and rhodium are known to have intermetallic substructures that range from clusters to three-dimensional networks. Bi12Rh3Cl2 contains intermetallic networks consisting of edge-sharing [RhBi8] cubes and antiprisms.
The researchers planned to “pull” the halogen atoms out without destroying the intermetallic regions under gentle conditions using an n-butyllithium solution. The chloride ions were extracted just as the scientists hoped, even though they seemed to be tightly enclosed by the narrow channels of the intermetallic network. Even more surprisingly, the resulting voids in the crystal structure were filled by bismuth atoms. The bismuth atoms came from barely noticeable chemical decomposition of the surface of the crystal.
The resulting product is Bi12Rh3Bi2, a metastable superconductor with a structure identical to that of the subchloride. During the reaction, the morphology of the crystal remains unchanged. “The transformation must be based on efficient transport of chloride ions out and bismuth ions into the network,” says Ruck. Crystallographic studies revealed a small change in the torsion angle of the [RhBi8] antiprisms. “The antiprisms act as hinges in the network,” explains Ruck. “Transient changes in the angle allow wide diffusion pathways to open up parallel to all of the intermetallic strands. Since the diffusion paths intersect, the transport system is three-dimensional.”
Although the intermetallic network only changes very slightly, the electronic properties are significantly different: the subchloride only demonstrates metallic conductivity along special directions that are insulated by nonconducting parts of the structure. In the intermetallic compound, in contrast, the conducting strands are metallically connected through the additional bismuth atoms. They are thus electrically connected, resulting in a three-dimensional metal.
About the Author
Professor Dr. Michael Ruck conducts research and teaches chemistry and food chemistry at the Technical University of Dresden. He works in the area of solid-state chemistry and is particularly interested in metallic compounds and low-temperature synthesis of materials. He is also a Fellow of the Max Planck Institute of the Chemical Physics of solids in Dresden.
Author: Michael Ruck, Technische Universität Dresden (Germany), http://www.cpfs.mpg.de/
Title: The Topochemical Pseudomorphosis of a Chloride into a Bismuthide
Angewandte Chemie International Edition, Permalink to the article: http://dx.doi.org/10.1002/anie.201309460
Media Contact
More Information:
http://pressroom.angewandte.orgAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

“Nanostitches” enable lighter and tougher composite materials
In research that may lead to next-generation airplanes and spacecraft, MIT engineers used carbon nanotubes to prevent cracking in multilayered composites. To save on fuel and reduce aircraft emissions, engineers…

Trash to treasure
Researchers turn metal waste into catalyst for hydrogen. Scientists have found a way to transform metal waste into a highly efficient catalyst to make hydrogen from water, a discovery that…

Real-time detection of infectious disease viruses
… by searching for molecular fingerprinting. A research team consisting of Professor Kyoung-Duck Park and Taeyoung Moon and Huitae Joo, PhD candidates, from the Department of Physics at Pohang University…





















