Molecular high-speed Origami – Researchers elucidate important mechanism of protein folding
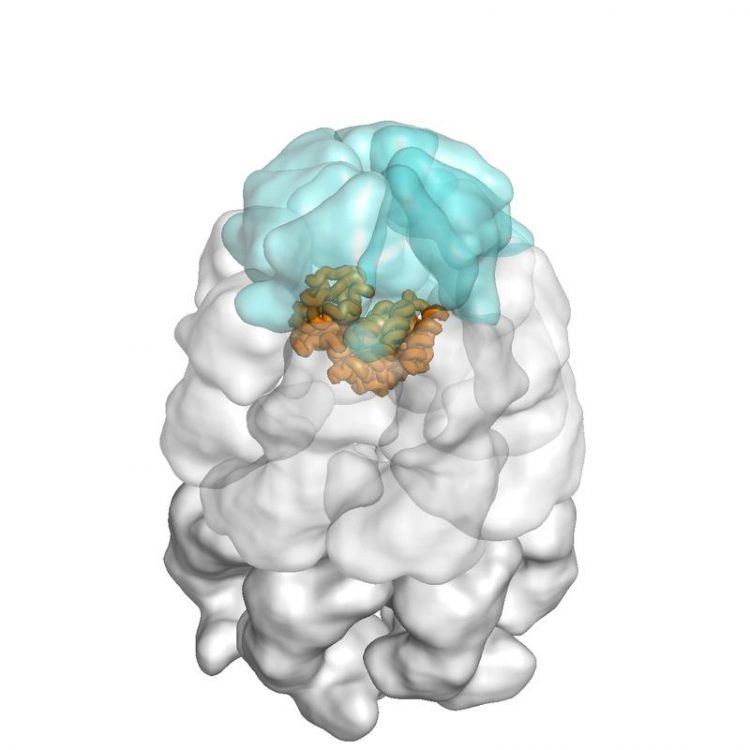
GroEL/ES nano-cage (light blue and white) with encapsulated substrate protein (orange). Image: Andreas Bracher / Copyright: MPI of Biochemistry
Scientists at the Max Planck Institute of Biochemistry (MPIB) have recently discovered a so far unknown sequence of reactions which is necessary for newly generated proteins to acquire their correct structure.
„In the mechanism we found, the folding is accomplished in a number of fast intermediate steps rather than in one single block“, explains Manajit Hayer-Hartl, MPIB research group leader. „Because this mode of action is energetically more favorable, the proteins are folded not only correctly, but also much faster than previously assumed.“
Proteins are the workhorses of the cell and thus responsible for almost all biological functions including metabolism, signal transmission or the determination of the cell’s shape. However, before they can fulfill their various tasks, the chain-like molecules must first adopt an intricate three-dimensional conformation. This process is called protein folding and is one of the most important processes in biology.
In fact, in the event of improper folding, proteins are often no more able to carry out their duties, or even tend to clump together in aggregates. This in turn can lead to severe diseases like Alzheimer’s or Parkinson’s. In order to avoid this, specialized proteins, the so-called chaperones, help other proteins to adopt their proper shape.
The bacterial chaperones GroEL and GroES serve as an example for this principle: together, they build up a cage-like structure in which they encapsulate new, not yet folded proteins, thereby al-lowing them to fold properly. However, the exact way in which this is accomplished has so far been unclear and is a research topic of the MPIB team led by Manajit Hayer-Hartl and F. Ulrich Hartl, in collaboration with John Engen from Northeastern University in Boston.
Active acceleration of folding
„Our results demonstrate that the chaperones not only prevent protein clumping, but also dramatically accelerate the folding process”, explains Florian Georgescauld, scientist at the MPIB. „Surprisingly, the chaperones achieve this by changing the mechanism of folding: Instead of folding in one large single block, the protein gets its final structure in a series of small, rapid steps – like an elaborate high-speed Origami.” The researchers think that splitting up the reaction might render it energetically more favorable, which in turn would lead to increased speed. Hence, the folding process is finished in a few seconds rather than in several minutes.
The study shows for the first time that chaperones can act not only passively, by preventing aggregation, but as an active folding cage that catalyzes the folding process. This results in a high-speed folding mechanism which is of particular biological relevance, so the researchers say, since in this way proteins can be folded faster than they are produced. Thus, a backlog of proteins which are not yet or improperly folded and the disastrous consequences which might go along with this can be avoided.
[HS]
Original Publication:
F. Georgescauld, K. Popova, A. J. Gupta, A. Bracher, J. R. Engen, M. Hayer-Hartl and F. U. Hartl: GroEL/ES Chaperonin Modulates the Mechanism and Accelerates the Rate of TIM-Barrel Domain Folding. Cell, May 8, 2014.
DOI: 10.1016/j.cell.2014.03.038
Contact:
Dr. Manajit Hayer-Hartl
Chaperonin-assisted Protein Folding
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
Germany
E-Mail: mhartl@biochem.mpg.de
http://www.biochem.mpg.de/hayer-hartl
Anja Konschak
Public Relations
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
Germany
Tel. +49 89 8578-2824
E-Mail: konschak@biochem.mpg.de
http://www.biochem.mpg.de/news
http://www.biochem.mpg.de/news/ueber_das_institut/forschungsbereiche/strukturforschung/hayer_hartl_press – Press Page of the Research Group “Chaperonin-assisted Protein Folding” (Manajit Hayer-Hartl)
http://www.biochem.mpg.de/en/rg/hayer-hartl – Website of the Research Group “Chaperonin-assisted Protein Folding” (Manajit Hayer-Hartl)
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















