Metal Strengthens Double Bond
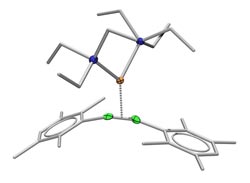
A double bond between two boron atoms (green) is strengthened due to the influence of platinum (orange): Simplified structure of the platinum-diboranyl complex.<br><br>Picture: Alexander Damme<br>
Professor Holger Braunschweig and his team come up with stunning new discoveries in chemistry with great regularity. This time, the Würzburg researchers turn an established model describing catalytic processes on its head.
Be it margarine, chemical fertilizers or plastic cups: The chemical principle of catalysis plays an important role in the production of various products. In the production process, a so-called catalyst enables certain reactions to proceed. Catalysts are indispensable for hardening vegetable oils into margarine or for producing polyethylene and other plastics.
Take the example of margarine: In order to create spreadable fat from liquid vegetable oil, you need to break bonds in hydrogen molecules. This is where a metallic catalyst comes in. Its metal atom pushes electrons into the bonds, destabilizing them in the process, so that they are ready for the desired reaction.
Established model turned on its head
A metal donates electrons, thereby weakening chemical bonds: This effect – known as the “Dewar-Chatt-Duncanson model” – has been known to chemists since 1953. However, the model must now be supplemented, having been turned on its head by chemists of the University of Würzburg.
The new insight: The electrons of a metal can also strengthen a chemical bond – at least in the case of a double bond between two boron atoms. This is reported in the journal “Nature Chemistry” by researchers of Professor Holger Braunschweig's study group.
Theory experimentally confirmed
A double bond between two boron atoms can accommodate exactly two additional electrons. Chemists speak in this context of a “free II-orbital”. If you fill this space, the bond should become stronger: This is the assumption that the Würzburg chemists Dr. Rian Dewhurst and Dr. Alfredo Vargas started from. They modeled their idea on the computer and found it confirmed – purely theoretically at first.
The next step was to confirm the theory by means of an experiment. Within the study group, the researchers found a molecule that was ideally suited for this purpose: a so-called platinum diboranyl complex. This molecule had been synthesized in a sophisticated process by Alexander Damme when working on his doctoral thesis.
Boron-boron double bond plus platinum
The centerpiece of the complex consists of two boron atoms that are linked to each other by a single bond in close proximity to a platinum atom. Damme devised the following procedure: He forced additional electrons on the complex, thus producing a boron-boron double bond.
According to the established model, this double bond should have been weaker than a “normal” boron-boron double bond due to the influence of the platinum metal. In actual fact, however, the bond even proved to be stronger. This was shown in a single crystal X-ray diffraction analysis of the material. This method allows you to determine how far the atoms of a molecule are apart from each other. The closer they are together, the stronger their bond will be. The Würzburg chemists found out that two boron atoms in a double bond come significantly closer together in the presence of platinum than they do without the metal.
New knowledge for textbooks
What are the consequences of this discovery? The everyday practice in chemical laboratories and industrial processes won't be affected for now. But the chemistry textbooks need to be supplemented. To be sure, the “Dewar-Chatt-Duncanson model” has not yet become obsolete; it remains applicable to carbon compounds. But it needs to be substantially extended now. You never know – maybe a model by the name of “Braunschweig-Damme-Dewhurst-Vargas” will be added.
“Bond-strengthening II backdonation in a transition-metal II-diborene complex”, Holger Braunschweig, Alexander Damme, Rian D. Dewhurst, and Alfredo Vargas, Nature Chemistry, 2012 Dec 9, DOI: 10.1038/NCHEM.1520
Contact person
Prof. Dr. Holger Braunschweig, Institute for Inorganic Chemistry of the University of Würzburg, T +49 (0)931 31-85260, h.braunschweig@uni-wuerzburg.de
Media Contact
More Information:
http://www.uni-wuerzburg.deAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















