Intimate insights into nature’s photosynthetic powerhouse
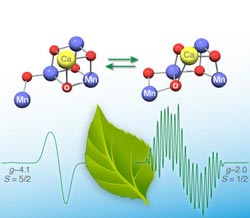
Figure (copyright by MPI CEC): The two structures of the core of nature's water oxidizing catalyst Photosystem II, which interconvert by changing bonds between an oxygen and its two manganese bondingpartners; a different spectroscopic signal is produced by eacharrangement. <br>
With the aid of quantum chemistry they were able to provide unexpected insight into the properties of the oxygen evolving complex (OEC). The OEC is the catalyst in plants that splits water using sunlight in order to build carbohydrates, thus powering all life on earth.
Its precise structure, which was showing enigmatic spectroscopic behaviour, could now finally be solved with the aid of quantum chemistry. In one of its most studied oxidation states the OEC revealed two types of spectroscopic signals.
These signals could be converted to one another by various treatments, but not in any structurally comprehensible way. Moreover the signals are so complex that a detailed molecular structure could not be deduced. With the aid of theoretical spectroscopic techniques, Dr. Dimitrios Pantazis, scientist at the MPI CEC, and his colleagues were able to show that the two signals are caused by two energetically similar and interconvertible structures of the complex.
The core of the enzyme consists of a partial cubic structure made of manganese, calcium and oxygen (Mn4CaO5 s. figure). “Calculations show, that the two structures differ only by one bond, that swaps between the central oxygen and the two terminal manganese atoms”, states Pantazis. This small change has a huge impact on the electronic structure and thus the spectroscopic properties of the molecule. Both structures are almost equal in energy and the bond swapping can happen over a low energetic barrier. Crucially, the scientists at the MPI additionally proved using theoretical simulations that each of the two structures has a distinct spectroscopic signature and that these two signatures have a one-‐to-‐one correspondence with the experimentally observed spectroscopic signals.
The deep understanding of the OEC is fundamental in order to further elucidate nature´s mysteries on the oxidation of water, a reaction that plays an essential role for energy research, such as in artificial photosynthesis. After these striking findings, research by Pantazis and his group is currently focused on identifying whether the oxygen atom swapping bonds with the manganese is one of the oxygen atoms released from the enzyme as molecular oxygen.
The new findings will shed light on the kinetics and exchange of water molecules that take part in the reaction, paving the way for a detailed atomic-‐level understanding of the mechanism of water oxidation.
Published online in Angewandte Chemie International Edition, August 21 http://dx.doi.org/10.1002/anie.201204705
The Max Planck Institute for Chemical Energy Conversion (MPI CEC) in Muelheim an der Ruhr focuses on fundamental chemical reactions that play a role for the storage and conversion of energy. The main objective is to save the energy of sunlight in small, energy rich molecules, and thus make it easily available independently of time and location. In the three departments Heterogeneous Reactions, Molecular Theory and Spectroscopy and Biophysical Chemistry work 75 scientists from more than 20 countries and with their expertise they contribute to a sustainable energy concept.
Media Contact
More Information:
http://www.cec.mpg.deAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Combatting disruptive ‘noise’ in quantum communication
In a significant milestone for quantum communication technology, an experiment has demonstrated how networks can be leveraged to combat disruptive ‘noise’ in quantum communications. The international effort led by researchers…

Stretchable quantum dot display
Intrinsically stretchable quantum dot-based light-emitting diodes achieved record-breaking performance. A team of South Korean scientists led by Professor KIM Dae-Hyeong of the Center for Nanoparticle Research within the Institute for…

Internet can achieve quantum speed with light saved as sound
Researchers at the University of Copenhagen’s Niels Bohr Institute have developed a new way to create quantum memory: A small drum can store data sent with light in its sonic…





















