Insect Gut Microbe with a Molecular Iron Reservoir
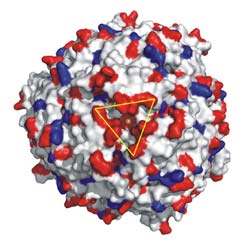
Molecular structure of the enzyme N-acyl amino acid hydrolase (AAH) from Microbacterium arborescens: Representation of the protein surface with negative (red) and positive (blue) charges. The triangle marks the iron uptake pore. Kornelius Zeth, MPI Tuebingen<br>
Iron plays an important role in almost every life form. Low iron can lead to deficiency symptoms and reduced growth, whereas too much iron may harm biomolecules like DNA. Max Planck researchers from Jena and Tuebingen have now elucidated the spatial structure of a bacterial enzyme in Microbacterium arborescens which is able to accumulate several hundred iron ions in its center – depending on the iron supply situation in its environment: for example in the larval gut of the Beet Armyworm Spodoptera exigua.
With its additional peroxidase activity the enzyme inhibits the occurrence of cell-damaging oxygen radicals. Moreover, it catalyzes the hydrolysis and formation of N-acyl glutamines (conjugates of the amino acid glutamine with fatty acids). The plant recognizes the larval pest with the help of these conjugates and initiates its chemical defense against invader. Structurally related enzymes are produced by other bacteria and are collectively termed as DNA protecting proteins under starvation (DPS). (The Journal of Biological Chemistry. DOI: 10.1074/jbc.M111.246108)
Microbes are omnipresent on earth. They are found as free-living microorganisms as well as in communities with other higher organisms. Thanks to modern biological techniques we are now able to address the complex communities and study the role of individual microorganisms and enzymes in more detail.
Microbacterium arborescens is a bacterium, which can be found in the guts of herbivorous caterpillars. The Department of Bioorganic Chemistry at the Max Planck Institute for Chemical Ecology studies interactions between insects and microorganisms which live in their digestive system. What is the advantage for both, insects and microbes? How strongly do they depend on each other? Do microbes play a role in mediating interactions between herbivorous insects and host plants? In the course of the experiments to answer these questions the scientists came across an enzyme they had isolated from M. arborescens, a resident in the gut of the Beet Armyworm Spodoptera exigua. It was called N-acyl amino acid hydrolase (AAH) because of its catalytic function: it catalyzes the synthesis and hydrolysis of conjugates of the amino acid glutamine with fatty acids. The N-acyl glutamines enter the infested plant via oral secretions and intestinal contents of the larvae and trigger the plant's defense responses. After cloning and sequencing the AAH encoding gene the scientists discovered an interesting result: AAH is closely related to proteins from other microorganisms: the “DNA protection during starvation (DPS)” proteins, which bind to DNA molecules and protect them by crystallization or by removal of dangerous OH• radicals. Jelena Pesek, PhD student in the Department of Bioorganic Chemistry at the institute, was surprised that the enzyme AAH from M. arborescens differs from DPS enzymes in other microbes to the effect that it additionally regulates the concentration of N-acyl glutamine (conjugates of glutamic acid with fatty acids) in the gut, which are important for molecular plant-insect interactions. Moreover, the enzyme is able to store Fe(III)ions in its center. If free Fe(II) is present, hydrogen peroxide (H2O2), which is synthesized by the insect's intestinal cells to fend off microorganisms, is converted to highly reactive hydroxyl radicals. The process is known as the Fenton's Reaction:
Fe2+ + H2O2 → Fe3+ + OH- + •HO (Fenton's Reaction)
The highly reactive hydroxyl radical •HO damages especially the DNA and thus causes dangerous mutations of the genetic material. In cooperation with Kornelius Zeth from the Max Planck Institute for Developmental Biology in Tuebingen the researchers succeeded in analyzing the iron transport mechanisms by means of crystallization and X-ray structure determination.
The protein consists of 12 identical subunits and has a molecular mass of 204 kDa – a considerable size for a single enzyme. The homo-oligomer is round and hollow inside. It can store up to 500 iron atoms as ferric iron (usually in the form of Fe2O3) in the hollow cavity. The iron transport into the cavity is unique: The spherical protein has four selective pores which provide access only to ferrous iron ions along with their hydration shells (six water molecules). At catalytic ferroxidase centers inside the cavity the Fe(II) is oxidized to Fe(III) with simultaneous reduction of the dangerous H2O2 to water (H2O).
The scientists assume that AAH ensures survival of M. arborescens in the larval gut, where conditions may be harsh and constantly changing depending on food quality. The enzyme is protective against oxidative stress, reducing the concentration of free Fe(II) by storing it and simultaneously neutralizing H2O2 as a source for cell damaging radicals. The evolutionary context of these processes as well as the formation and hydrolysis of N-acyl glutamines which are also catalyzed by AAH are still unknown. Because of their detergent character these compounds may help the larvae to better digest the plant food. In the course of evolution, attacked host plants may have “learned” to exploit the conjugates which enter the leaves during herbivory as a chemical alarm signal in order to activate their defense against the insect pest efficiently. [JWK/AO]
Original Publication:
Jelena Pesek, Rita Büchler, Reinhard Albrecht, Wilhelm Boland, Kornelius Zeth: Structure and Mechanism of Iron Translocation by a Dps Protein from Microbacterium arborescens. The Journal of Biological Chemistry 286. DOI: 10.1074/jbc.M111.246108
Further Information:
Prof. Dr. Wilhelm Boland, Tel.: 03641 – 57 1201, boland@ice.mpg.de
Dr. Kornelius Zeth, Tel.: 07071-601323, kornelius.zeth@tuebingen.mpg.de
Picture Requests:
Downloads: http://www.ice.mpg.de/ext/735.html
or contact Angela Overmeyer, Max Planck Institute for Chemical Ecology, Hans-Knöll-Straße 8, 07745 Jena, Germany. Tel.: +49 (0)3641- 57 2110; overmeyer@ice.mpg.de
Media Contact
More Information:
http://www.ice.mpg.deAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Making diamonds at ambient pressure
Scientists develop novel liquid metal alloy system to synthesize diamond under moderate conditions. Did you know that 99% of synthetic diamonds are currently produced using high-pressure and high-temperature (HPHT) methods?[2]…

Eruption of mega-magnetic star lights up nearby galaxy
Thanks to ESA satellites, an international team including UNIGE researchers has detected a giant eruption coming from a magnetar, an extremely magnetic neutron star. While ESA’s satellite INTEGRAL was observing…

Solving the riddle of the sphingolipids in coronary artery disease
Weill Cornell Medicine investigators have uncovered a way to unleash in blood vessels the protective effects of a type of fat-related molecule known as a sphingolipid, suggesting a promising new…





















