Viral infections: Identifying the tell-tale patterns
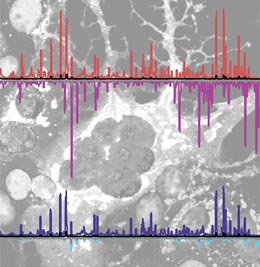
Interaction profiles of cellular RNA sensors with RNA strands of the measles virus genome (superimposed on a micrograph showing cells infected with measles virus).
When viruses infect cells, they take control of cellular metabolism and hijack cellular resources for the production of viral proteins. This process is dependent on viral RNA molecules that are delivered directly to (in the case of RNA viruses) and/or newly synthesized in the host cell, and provide the blueprints for the fabrication of viral proteins by the cell’s translational apparatus.
However, cells possess defense systems that are activated by specialized sensors that can distinguish viral RNAs from host RNAs. These proteins, three of which belong to the family of RIG-I like receptors (or RLRs), recognize and bind specifically to foreign RNAs. This in turn alerts the innate immune system, which proceeds to destroy the foreign RNAs, thus preventing the production of new virus particles.
“Based on in-vitro experiments, it is known that RLR proteins bind to certain characteristic patterns in viral RNAs, but it had not been possible to isolate the precise RNA sequences bound by these proteins in living, virus-infected cells,” says Professor Karl-Peter Hopfner of LMU’s Genzentrum.
Tethering RNA to proteins with UV light
Hopfner, in collaboration with his colleagues Karl-Klaus Conzelmann (LMU), Johannes Söding (LMU) and Adolfo García-Sastre (Mount Sinai Hospital, New York), made use of a clever experimental strategy to get around this problem, which enabled them to purify and characterize ribonucleoprotein complexes containing viral RNAs from virus-infected cells.
The intrinsic stability of the interaction between RLRs and viral RNAs is very low. So the researchers first had to stabilize the complexes in order to isolate them intact. For this purpose, they infected cells with measles virus, and incubated them in the presence of a chemically modified, photoactivatable RNA precursor, which is incorporated into newly synthesized viral RNAs. “Provided that the physical distance between an RNA and its binding protein is short enough, subsequent exposure of such cells to UV light induces the formation of a stable covalent bond between them,” Hopfner explains.
The resulting RNA-protein complexes could then be isolated from the cells and, after detachment of the proteins, the nucleotide sequences of the RNAs could be determined. “This allowed us to determine how RLRs recognize foreign RNAs and how the latter differ from endogenous cellular RNAs,” says Hopfner.
The researchers found that the RLR proteins RIG-I and MDA5 indeed recognize defined elements within viral RNAs in living cells that have been infected by measles virus. Like many other viruses, including the one that causes rabies, the measles virus possesses a single-stranded RNA genome. Unlike DNA viruses, it therefore delivers an RNA template directly into the host cell. However, this molecule must then be transcribed by its associated viral RNA polymerase to generate the mRNAs required for synthesis of viral proteins and propagation of the infection.
Sensors bind to specific regions
“And while RIG-I preferentially binds to certain sequence patterns found at the exposed ends of different viral RNAs both in vitro and in vivo, MDA5 rather surprisingly recognizes not the viral genome itself, but apparently certain regions located within viral mRNAs,” Hopfner explains. Moreover, these regions differ in their base composition from sequences found in other viral RNAs, suggesting that MDA5 relies on these structural differences to discriminate between viral and endogenous RNAs.
Hopfner and his team now plan to investigate the interaction of RLRs with other viral nucleic acids, in order to obtain a clearer picture of the molecular mechanisms that enable these proteins to detect foreign RNAs. This should in turn shed light on why the innate immune system has difficulty in responding to particular viruses, and how RLR-associated autoimmune diseases such as rheumatoid arthritis arise. A better understanding of both of these issues could suggest new approaches to the treatment of both viral infections and autoimmunity.
(PloS Pathogens 2014)
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















