Hunting pathogens at full force
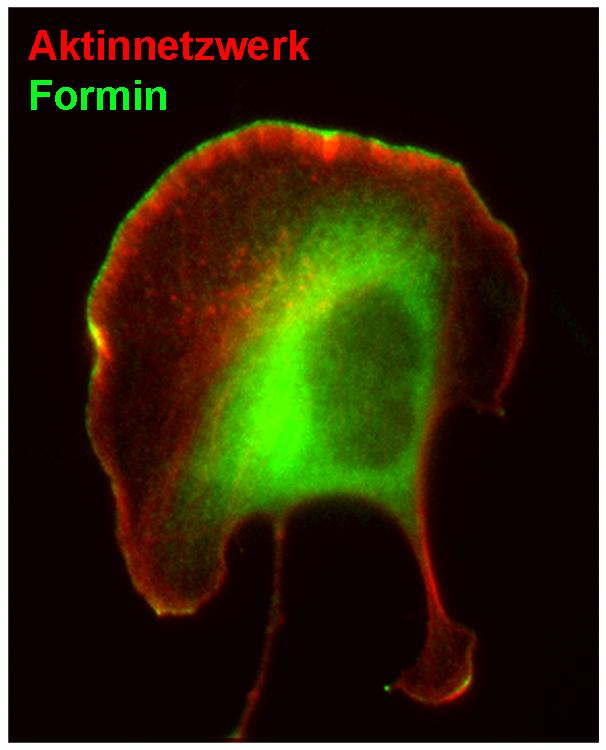
Migrating melanoma cell whose motion towards the top left is supported by formin proteins (green). HZI/Frieda Kage
Cells inside the human body contain a flexible polymer network called the cytoskeleton which consists of actin filaments – and other components – and undergoes constant assembly and disassembly. This turnover of actin filaments allows cells to change shape and move. Such movements are important for example during embryonic development, wound healing but also for a properly operating immune system. To be able to move through tissue, cells need to expend energy and apply force.
For example, immune cells advance into all regions of our body to detect and fight pathogens. In turn, though, some pathogens can abuse the cytoskeleton to adhere to or penetrate into cells. Researchers of the Helmholtz Centre for Infection Research (HZI) and the Technische Universität Braunschweig have now elucidated the molecular mechanisms that allow cells to move forward effectively, using powerful cell edge protrusions.
By applying the CRISPR/Cas technique, a cutting-edge genome editing method, the researchers were the first to precisely define the roles of a specific protein family called formins in the establishment of densely-branched actin networks. The research was performed in close cooperation with Hannover Medical School (MHH) and the University of Leipzig. The researchers published their results in the prestigious scientific journal, Nature Communications.
“When cells move through body tissues, they form various extensions on their front side: sheets of cytoplasm called lamellipodia and finger-like filopodia, but also bleb-like evaginations,” says Klemens Rottner, who is the head of the “Molecular Cell Biology” research group at the HZI and professor at the Zoological Institute of the Technische Universität Braunschweig. The cytoskeleton of the cells plays a crucial role in this process. Unlike the bony skeleton, it is not a rigid framework, but instead constitutes a dynamic meshwork composed of various types of polymeric filaments.
To change their shape and to spread, cells need specific micro-filaments – tiny fibres only nanometres in thickness that are made of the structural protein called actin. Cell motion is enabled by the interplay of actin filaments with special myosin proteins. “The actin-myosin filaments are basically the muscles of the cell,” says Rottner.
Viewed in detail, lamellipodia consist of transverse cross-linked actin filaments and parallel-aligned filament bundles that are enclosed by a thin cell membrane. These actin filaments are anchored at the tip of the lamellipodial extension and push it forward due to continuous addition of their individual building blocks, the so called actin monomers, while constantly disassembled at their rear. This continuous turnover of actin filaments allows the cell to move forward.
Previously, it was discovered that a certain protein complex is essential for the assembly of actin networks in lamellipodia – the Arp2/3 complex. However, the researchers have now characterized another important component: A family of proteins known as formins, which bind to and regulate the rapidly growing ends of actin filaments. “The role of these proteins in lamellipodia was unknown.
It was presumed that they would simply control the rate at which monomers are added to the filaments of the network,” says Rottner. However, the authors of the study discovered now that these proteins regulate mainly the filament density and stability of the actin network. Unlike the Arp2/3 complex, formins connect the growing actin filament ends to the cell membrane such that forces generated can directly be transferred to the membrane and an efficient forward motion of the cell be assured.
In their experiments, the research team used the CRISPR/Cas technology to knock-out formins both in tumour cells and in connective tissue cells of mice. “This extremely efficient and comparably inexpensive technology allowed us to eliminate the production of two formins with related functions simultaneously and completely,” says Frieda Kage, who is the lead author of the study and a scientist in Rottner's team. “As a result, we were able to establish that these formins are not essential for the formation of lamellipodia as such, since the actin network is formed anyway by the Arp2/3 complex,” the junior scientist adds. “But the network was much softer since both density and spatial arrangement of the filaments in the network were changed.”
Klemens Rottner summarises: “Only the combined activities of Arp2/3 protein complex and formins allows cells to form stable, densely-branched actin networks. In the absence of formins, cell motion is strongly impaired, mostly because the cells cannot move forward efficiently with underlying actin networks being too soft.”
The scientists were able to directly measure the propulsion forces of the cells with and without formins, which are in the nano-Newton range. For this, they used an atomic force microscope (AFM) in cooperation with Prof Josef Käs from the Institute for Experimental Physics I at the University of Leipzig. “We measured a reduction of propulsion forces by 75% in cells lacking formins,” says Rottner. “This drastic reduction of the forces exerted by these cells explains why they migrate only very slowly in the absence of these formins,” Kage adds.
Interestingly, these formins are closely associated with certain types of cancer such as carcinomas and melanomas: The amounts of formins present in patients suffering from aggressive tumours and metastases are often high. The data of the present study provide a molecular understanding for the reasons behind these observations.
“Since immune cells are among those cells that need to apply forces to reach pathogens, we think that the investigation of these motility processes will be crucial for fighting infectious diseases. We need to understand these processes in detail to be able to find new approaches for therapies,” says Klemens Rottner. “In addition, many bacteria and viruses make use of polymerisation forces generated by actin filaments, to either penetrate into cells, move through them or even into neighbouring, still non-infected cells.”
The results of the present study provide a starting point for future investigations using various pathogens such as Salmonella, Shigella or Listeria, since all of those are known to re-program the actin cytoskeleton and utilise the forces generated by this system to their own benefit. Specific manipulation of the formins investigated here, in particular during early phases of contact between pathogen and host cell, may constitute a promising approach to inhibit infections.
Original publication:
Kage, F. et al.: FMNL formins boost lamellipodial force generation. Nature Communications 2017; DOI: 10.1038/ncomms14832
The Helmholtz Centre for Infection Research:
Scientists at the Helmholtz Centre for Infection Research (HZI) in Braunschweig, Germany, are engaged in the study of different mechanisms of infection and of the body’s response to infection. Helping to improve the scientific community’s understanding of a given bacterium’s or virus’ pathogenicity is key to developing effective new treatments and vaccines. http://www.helmholtz-hzi.de/en
Contact:
Susanne Thiele, Press Officer
susanne.thiele@helmholtz-hzi.de
Dr Andreas Fischer, Editor
andreas.fischer@helmholtz-hzi.de
Helmholtz Centre for Infection Research
Press and Communications
Inhoffenstr. 7
D-38124 Braunschweig
Germany
Phone: +49 531 6181-1404
http://www.nature.com/articles/ncomms14832 – publication
https://www.helmholtz-hzi.de/en/news_events/news/view/article/complete/hunting_p… – press release
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















