How Natural Channel Proteins Move in Artificial Membranes
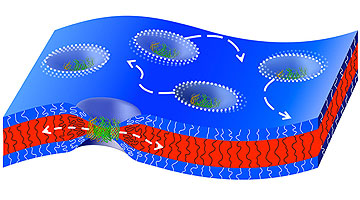
Natural channel proteins move sideways in a thick artificial membrane that condenses around the channel proteins. (Image: Reprinted with permission from ACS)
The membranes of the cells in our bodies are only approximately 4 to 5 nanometers thick and consist of a complex mixture of lipids and specific membrane proteins, which also include channel proteins. This kind of cell membrane can be described as a fluid 2-D solution, in which the components are able to move laterally. These movements within the membrane are dependent on the flexibility and fluidity of the components and ultimately determine the functionality of the membrane.
Dynamic channel proteins
Chemists at the National Center of Competence in Research (NCCR) Molecular Systems Engineering working under Professor Wolfgang Meier and Professor Cornelia Palivan from the University of Basel have now integrated three different channel proteins into artificial membranes of 9 to 13 nanometers in thickness and have measured their movements for the first time.
The researchers began by creating large membrane models with embedded and dyed channel proteins; they then put them on a glass surface and measured them using a single-molecule measuring method known as fluorescence correlation spectroscopy. All three channel proteins were able to move freely within the membranes of various thicknesses – this took up to ten times longer than in the lipid bilayers of their natural environment.
Flexibility is a necessity
In thicker membranes, the building blocks of the membrane (polymers) must be able to condense around the channel proteins in order to alter their fixed size. To do so, the membrane building blocks have to be sufficiently flexible. This had already been described in theory.
The researchers at the University of Basel have now been able to measure this in a practical experiment for the first time, demonstrating that the thicker the membrane, the slower the movement of the channel protein is in comparison to the movement of the actual polymers that form the membrane.
“This phenomenon is effectively a local decrease in fluidity caused by condensation of the polymers,” explains lead author Fabian Itel. In essence, however, the behavior of the channel proteins in the artificial membranes is comparable to that in their natural environment, the lipid bilayer, with the time scale of the movements being approximately ten times lower. The research project received funding from the Swiss National Science Foundation and the NCCR Molecular Systems Engineering.
Original article
Fabian Itel, Adrian Najer, Cornelia G. Palivan, and Wolfgang Meier
Dynamics of membrane proteins within synthetic polymer membranes with large hydrophobic mismatch
Nano Letters (2015), doi: 10.1021/acs.nanolett.5b00699
https://www.unibas.ch/en/News-Events/News/Uni-Research/How-Natural-Channel-Prote…
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















