How cone snail venom minimizes pain
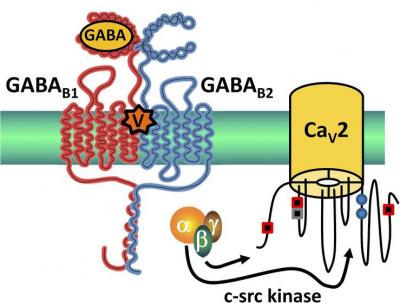
This schematic shows the proposed mechanism by which the cone snail venom Vc1.1 reduces pain sensation through indirect inhibition of R-type (Cav2.3) neuronal voltage-gated calcium channels. Credit: Rittenhouse, 2014
The venom from marine cone snails, used to immobilize prey, contains numerous peptides called conotoxins, some of which can act as painkillers in mammals. A recent study in The Journal of General Physiology provides new insight into the mechanisms by which one conotoxin, Vc1.1, inhibits pain.
The findings help explain the analgesic powers of this naturally occurring toxin and could eventually lead to the development of synthetic forms of Vc1.1 to treat certain types of neuropathic pain in humans.
Neuropathic pain, a form of chronic pain that occurs in conjunction with injury to—or dysfunction of—the nervous system, can be debilitating and difficult to treat, and the medical community is eager to find better methods to minimize what can be a serious condition.
Neuropathic pain is associated with changes in the transmission of signals between neurons, a process that depends on several types of voltage-gated calcium channels (VGCCs). However, given the importance of these VGCCs in mediating normal neurotransmission, using them as a pharmacological target against neuropathic pain could potentially lead to undesirable side effects.
In previous studies, David Adams and colleagues from RMIT University in Melbourne showed that Vc1.1 acted against neuropathic pain in mice; they found that, rather than acting directly to block VGCCs, Vc1.1 acts through GABA type B (GABAB) receptors to inhibit N-type (Cav2.2) channels.
Now, Adams and colleagues show that Vc1.1 also acts through GABAB receptors to inhibit a second, mysterious class of neuronal VGCCs that have been implicated in pain signaling but have not been well understood—R-type (Cav2.3) channels. Their new findings not only help solve the mystery of Cav2.3 function, but identify them as targets for analgesic conotoxins.
Article: Berecki, G., et al. 2014. J. Gen. Physiol. doi: 10.1085/jgp.201311104
Commentary: Rittenhouse, A.R. 2014. J. Gen. Physiol. 10.1085.jgp.201411190
About The Journal of General Physiology
Founded in 1918, The Journal of General Physiology (JGP) is published by The Rockefeller University Press. All editorial decisions on manuscripts submitted are made by active scientists in conjunction with our in-house scientific editor. JGP content is posted to PubMed Central, where it is available to the public for free six months after publication. Authors retain copyright of their published works and third parties may reuse the content for non-commercial purposes under a creative commons license. For more information, please visit http://www.jgp.org.
Research reported in the press release was supported by the National Health and Medical Research Council.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A new look at the consequences of light pollution
GAME 2024 begins its experiments in eight countries. Can artificial light at night harm marine algae and impair their important functions for coastal ecosystems? This year’s project of the training…

Silicon Carbide Innovation Alliance to drive industrial-scale semiconductor work
Known for its ability to withstand extreme environments and high voltages, silicon carbide (SiC) is a semiconducting material made up of silicon and carbon atoms arranged into crystals that is…

New SPECT/CT technique shows impressive biomarker identification
…offers increased access for prostate cancer patients. A novel SPECT/CT acquisition method can accurately detect radiopharmaceutical biodistribution in a convenient manner for prostate cancer patients, opening the door for more…





















