Hot on the trail of metabolic diseases and resistance to antibiotics
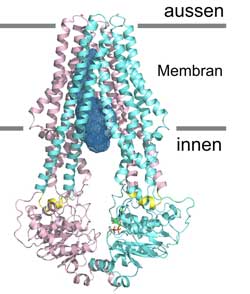
Detailed side-view of the ABC transporter “TM287/288”. The transporter adopts is inward-facing state. The two different protein chains are marked in turquoise and pink. Picture: UZH<br>
Biochemists from the University of Zurich and the NCCR Structural Biology have succeeded in determining the atomic structure of a new ABC transporter. The insights gained could give rise to new therapies to treat multi-resistant bacteria, cystic fibrosis or gout, for instance.
ABC transporters are membrane proteins that actively pump a wealth of molecules across the membrane. Over 40 different ABC transporters perform vital functions in humans. Genetic defects in ABC transporters can trigger metabolic diseases such as gout, neonatal diabetes or cystic fibrosis, and certain ABC transporters also cause resistance to a wide range of drugs. In tumor cells, increased amounts of ABC transporters that pump chemotherapeutic substances out of the cell are often produced, thus rendering anticancer drugs ineffective. Analogous mechanisms play a key role in many pathogenic bacteria: ABC transporters carry antibiotics out of the cell – multi-resistant bacteria are the result.
Despite their major importance in biology and medicine, so far the atomic structure of only a few ABC transporters has been decoded. Now, under the supervision of Markus Seeger and Professor Markus Grütter, PhD student Michael Hohl and senior scientist Christophe Briand have succeeded in cracking the atomic structure of the new ABC transporter “TM287/288”.
Illuminating asymmetry
The membrane protein originates from a thermophilic bacterium. Compared to structures already known, “TM287/288” has two different protein chains that assemble into a heterodimer. About half of the 40 human ABC transporters are heterodimers. “The asymmetries discovered enable us to consider the role of ABC transporters in a new light,” explains Seeger. “In the longer term, our results could help develop new medication against multi-resistant bacteria or tumors that are difficult to treat. They also make new approaches to curing or alleviating hereditary diseases possible,” concludes Grütter.
Literature:
Michael Hohl, Christophe Briand, Markus G. Grütter & Markus A. Seeger. Crystal structure of a heterodimeric ABC transporter in its inward-facing conformation. In: Nature Structural & Molecular Biology, March 28, 2012. Doi: 10.1038/nsmb.2267
Contact:
Dr. Markus Seeger
Department of Biochemistry
University of Zurich
Tel.: +41 44 635 55 52
Email: m.seeger@bioc.uzh.ch
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















