From Termite Fumigant to Molecular Coupling
The coupling of molecular building blocks nearly as easy as “snapping” them together can be realized by means of the “click chemistry” tool kit. American scientists have now introduced another achievement for the click concept in the journal Angewandte Chemie: the sulfur fluoride exchange reaction (SuFEx) can be used to form robust inorganic bridges between carbon centers and opens up a fully unexplored area of chemistry with countless new molecules that could form the basis for new drugs, diagnostics, plastics, “intelligent” materials, and many other products.
Developed in the 1990s by Nobel Laureate K. Barry Sharpless and his colleagues, the concept of click chemistry is aimed at synthesizing target molecules rapidly and precisely from smaller units. The reactions must be specific, broadly applicable, and environmentally friendly while delivering high yields.
They must also be based on inexpensive, widely available reagents that react under mild and uncomplicated conditions. Since the discovery of the azide–alkyne cycloaddition reaction in 2002 by the Sharpless team, the click concept has become established as a universal chemical technique.
A team led by Sharpless at The Scripps Research Institute in La Jolla (CA, USA) has now developed another groundbreaking click reaction: sulfur fluoride exchange (SuFEx). This reaction exploits the very special reactivity of sulfur fluorides and makes it possible for chemists to bind together molecules of their choice.
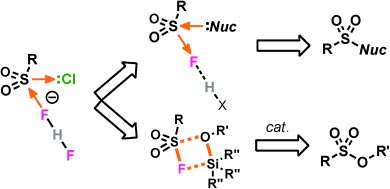
Like most click reactions, the process itself is an old one that has been improved to allow the previously underestimated sulfate bond to be used as a universally applicable connector for linking a variety of molecular building blocks.
The starting material is a common, commercially available chemical called sulfuryl fluoride (SO2F2) that is widely used as a fumigant against termites and other pests. It was previously considered generally inert—incorrectly, as Sharpless and his co-workers have found. The team was able to make this chemical reactive in a reliable and predictable way.
In the SuFEx reaction, the fluoride ion must be extracted from a bond with a hexavalent sulfur atom. This is not so easy, so the SO2—F unit is remarkably stable in typical acidic or basic environments. This bond thus fulfills a central requirement of click chemistry: it remains “invisible” under most conditions, coming to life only on demand.
A broad palette of potential applications could benefit from this reaction. The teams of Sharpless and V. V. Fokin developed an efficient, nearly quantitative synthesis of high-molecular-weight polysulfate polymers that should be easy to implement on an industrial scale. Linked by sulfate groups, these polymers are sulfur-containing analogues of polycarbonates and represent a new class of plastics potentially superior to present-day materials.
One particular advantage is that unlike polycarbonates, which can react with water to give off bisphenol A—a substance that has hormonelike properties and poses problems for both health and the environment—polysulfates are resistant to hydrolysis and thus cannot release monomers.
This is just one application for the SuFEx reactions; many other reactions with other building blocks are possible. An advantage for the biological sciences is that sulfate links do not occur in any life forms and the new SuFEx reaction does not interfere with biological processes.
About the Author
K. Barry Sharpless, W. M. Keck Professor at The Scripps Research Institute and its Skaggs Institute for Chemical Biology, pursues and develops useful new chemical connectivity. Click chemistry was conceived by him in the mid-1990s as a method for rapidly discovering, and improving existing, useful reactivity. Now his group has found its 2nd 'perfect' click reaction. In 2001 he shared the Nobel Prize in Chemistry for his work on asymmetric catalysis.
Author: K. Barry Sharpless, The Scripps Research Institute, La Jolla (USA), http://www.scripps.edu/sharpless
Title: Sulfur(VI) Fluoride Exchange (SuFEx): Another Good Reaction for Click Chemistry
Angewandte Chemie International Edition 2014, 53, No. 35, 9430–9448, Permalink to the article: http://dx.doi.org/10.1002/anie.201309399
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















