Filming chemistry with a high speed x-ray camera
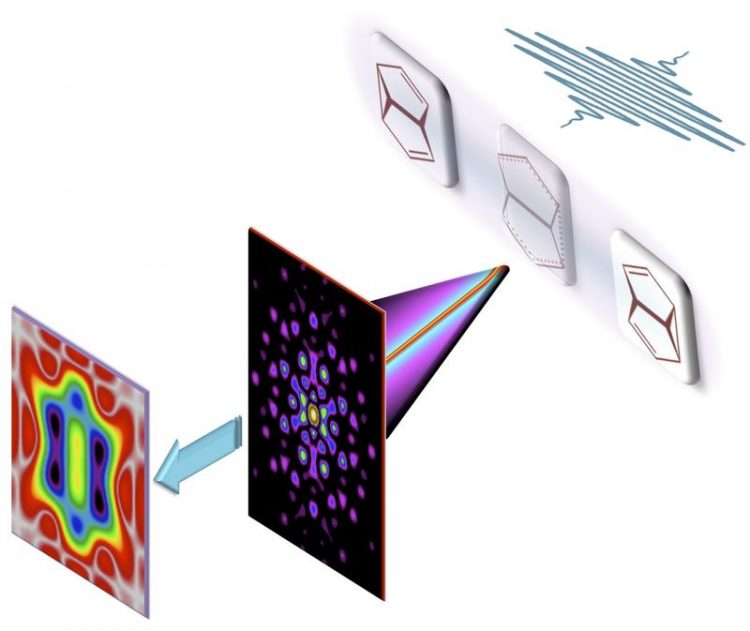
Filming bond making and bond breaking during a pericyclic reaction. (credit: MBI)
Scientists of the Max-Born-Institut (MBI) in Berlin were able to show theoretically that the ultrafast x-ray camera is not only sensitive to inert core electrons but may also visualize the motion of chemically active valence electrons.
While the motion of valence electrons is at the very heart of chemical reactions, only a small fraction among them participates actively. The valence electron charge transferred between the atoms is often just a fraction of the charge of an electron.
And those that do participate, do it very quickly: the duration of many very important chemical processes, such as first steps in vision and light harvesting, is measured in only tens to a hundred of femtoseconds – a femtosecond is a millionth of a billionth of a second. Making a movie of the chemically active electrons is therefore very challenging.
First, one needs a camera with exquisite temporal and spatial resolution. Second, one needs a very sensitive camera. Indeed, one would really like to see not just how the atoms move, but also how the new bonds are formed as the old ones are broken – and this means filming the few active valence electrons in the sea of all electrons attached to the many atoms in the molecule.
An X-ray camera easily fits the first requirement. X-ray scattering has been indispensable in studying the structure of matter with atomic-scale spatial resolution since the discovery of x-rays. Thanks to enormous technological progress, it is now becoming possible to generate ultrashort flashes of x-rays, adding femtosecond temporal resolution to structural sensitivity. These flashes of x-rays promise to provide stroboscopic snapshots of chemical and biological processes in individual molecules.
However, fitting the second requirement – the sensitivity to active valence electrons – has never been the strength of an x-ray camera. X-ray scattering is always dominated by core and inert valence electrons. The small fraction of valence electrons actively participating in a chemical reaction is generally presumed lost in the scattering signal, seemingly placing ultrafast x-ray imaging of these electron densities out of the realm of possibility
Our work, published in Nature Communications, suggests a way to resolve this challenge. In this work, we theoretically demonstrate a robust and effective method to extract the contributions of chemically active valence electrons from the total x-ray scattering by a single molecule – a critical step in the endeavor to film bond making and bond breaking as it happens, in space and time. Our paper shows how, by combining the standard analysis of the full x-ray scattering pattern with an additional analysis of the part of the scattering pattern, which is limited to relatively small momentum transfer, one nearly effortlessly brings to the fore the motion of chemically active valence electrons.
The work not only showed how to film chemically active valence electrons with x-rays, it has also provided an experimental access to the long-standing problem: Are the new bonds made at the same time as old bonds are broken, or is there a time-delay between these two processes?
The x-ray camera confirms that the answer depends on whether the atoms have enough energy to climb over the energy barrier, which separates reactants from products, or if they have to resort to the quantum trick of tunneling through the energy barrier the only option available when their energy is not sufficient to overcome it. In the first case we confirm a time-delay between the breaking of old and the formation of new bonds. In the second case, we see no delay: the new bonds are built in concert with the destruction of the old ones. We hope our work will bring new insights into ways to initiate and control complex chemical and biological reactions.
Original publication:
Timm Bredtmann, Misha Ivanov, Gopal Dixit
X-ray imaging of chemically active valence electrons during a pericyclic reaction
Nature Communication doi:10.1038/ncomms6589
Fig. 1: Filming bond making and bond breaking during a pericyclic reaction: We show theoretically that the ultrafast x-ray camera is not only sensitive to inert core electrons but may also visualize the motion of chemically active valence electrons. (credit: MBI)
Abb.: A combination of the standard analysis of the full x-ray scattering pattern (A, B) with an additional analysis of the part of the scattering pattern, which is limited to relatively small momentum transfer, one nearly effortlessly brings to the fore the motion of chemically active valence electrons during a pericyclic reaction (C, D). The breaking and making of chemical bonds along different reaction paths may thus be filmed and analyzed directly. (credit: MBI)
Contact
Dr. Timm Bredtmann Tel: 030 6392 1239
Prof. Misha Ivanov Tel: 030 6392 1210
Dr. Gopal Dixit Tel: 030 6392 1239
Max-Born-Institut (MBI)
im Forschungsverbund Berlin e.V
Max-Born-Straße 2A
12489 Berlin
GERMANY
Tel. ++49 30 6392 1505
Fax. ++49 30 6392 1509
Email: mbi@mbi-berlin.de
Weitere Informationen:
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















