Deciphering the mechanism of an ion pump
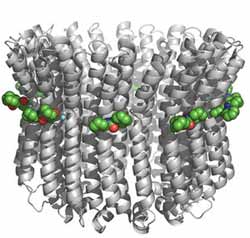
Figure 1: The crystal structure of the E. hirae V-ATPase with molecules of DCCD (green spheres) bound to E139 at each individual subunit. <br>Copyright : 2011 Takeshi Murata <br>
From an analysis of the sodium-transporting vacuolar ATPases (V-ATPases) of the bacterium Enterococcus hirae, Takeshi Murata of the RIKEN Systems and Structural Biology Center, Yokohama, and colleagues recently obtained valuable structural and functional information about a process that pumps protons and other positively charged ions across cellular membranes1.
Adenosine triphosphate (ATP) is the primary energy ‘currency’ within cells, and numerous enzymes are powered by the metabolic processing of this molecule via a mechanism known as hydrolysis. V-ATPases can exploit this process to pump positively charged ions across cellular membranes. This process occurs at the junction between a rotating ‘K’ domain and a fixed ‘a’ domain within the segment of the protein that resides at the cell membrane, although the specifics remain unclear.
N,N’-dicyclohexylcarbodiimide (DCCD), a chemical that selectively reacts with a specific glutamate amino acid (E139) within the sodium-binding pockets of the K ring, proved valuable in assessing this protein’s function. The researchers demonstrated that DCCD inhibited sodium binding by nearly 30-fold, but that this inhibition was sharply reduced when the enzyme was pretreated with sodium ions, suggesting that the two molecules interact with overlapping targets within the ring.
The K ring is composed of ten identical subunits, and DCCD efficiently reacts with E139 in each of these individual components (Fig. 1). By gathering structural data from the DCCD-treated V-ATPase, Murata and colleagues obtained a snapshot of what the protein looks like in the absence of sodium, which they could in turn compare against the structure of the sodium-bound form.
Although the two structures were largely similar, DCCD treatment triggered a change in E139 that locked the sodium binding sites into an ‘open’ structure that prevented ion retention. The negative charge of E139 made an important contribution to the binding of the positively charged Na+ ion; DCCD appeared to work by neutralizing this charge. The researchers hypothesize that a similar process governs ion release during the transport process; as the K domain rotates, each subunit’s E139 interacts with a positively charged amino acid on the domain, triggering ion release and transfer across the membrane.
Confirming this model will require additional structural data. “We would like to obtain the structure of [the] whole complex containing both the rotor ring and a-subunit,” Murata says. Nevertheless, these findings could prove immediately applicable to the development of more effective ATPase inhibitors, a class of drugs potentially useful for treating cancer and other diseases. “V-ATPases are of considerable pharmacological interest,” says Murata.
The corresponding author for this highlight is based at the Systems and Structural Biology Team, RIKEN Systems and Structural Biology Center
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















