Collapse of the Cellular Protein Network Causes Alzheimer’s?
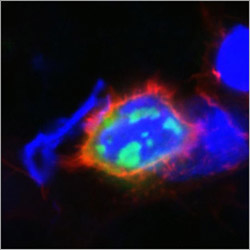
Toxic protein aggregates can cause neurodegenerative diseases (red: membrane of the cell, blue: interior of the cell, green: toxic aggregates).<br>Picture: Heidi Olzscha / Copyright: MPIB<br>
To fulfill their different functions, proteins have to acquire the correct three-dimensional structure. In other words, polypeptides have to fold first. Molecular chaperones, a diverse group of conserved proteins, have specialized to assist other proteins during their folding.
If the chaperones fail, misfolding and aggregation of the newly synthesized and pre-existing proteins might occur. In the worst case, this results then in neurodegenerative diseases, such as Alzheimer’s, Huntington’s chorea or Parkinson’s. Alzheimer’s disease, for example, develops because the A-beta and tau proteins aggregate, which leads to neuronal dysfunction and cell death. According to Alzheimer Forschung Initiative e. V., approximately 1.2 million people suffer from this disease only in Germany. The risk to fall ill grows with increasing age.
Scientists in the Department of Cellular Biochemistry at the Max Planck Institute of Biochemistry, headed by F.-Ulrich Hartl, now established a novel experimental model aimed at elucidating cellular protein misfolding and discovered why the misfolding and aggregation are deleterious for cells. They prepared several artificial aggregating proteins without any biological function and introduced them into cells. These model proteins clumped together, coaggregating many natural proteins and, in that way, disturbing their function. By means of quantitative proteomics, the researchers discovered that the affected proteins share certain structural characteristics which predispose them for the co-aggregation: They are large in size, less hydrophobic and show a significant increase of disorder in their structure.
“These are proteins that have not only many, but also very important functions in the cell”, explains Martin Vabulas. “For instance, they are responsible for the stability of the cytoskeleton, the organization of the chromatin in nucleus, the transcription of DNA to RNA or the synthesis of proteins. Simultaneous disturbance of several of these essential processes is most probably the reason of the cellular break-down. As a consequence, protein misfolding diseases develop.”
Molecular chaperones could possibly prevent this dire scenario. They are able to shield the aggregates, so that the aggregates cannot get in touch with other proteins anymore. The scientists hope that their new insights might help to develop novel therapeutic strategies in the battle against neurodegenerative diseases, especially at the earlier stages, before the irreversible collapse of cellular protein network sets in.
[UD]
Original Publication:
H. Olzscha, S. M. Schermann, A. C. Woerner, S. Pinkert, M. H. Hecht, G. G. Tartaglia, M. Vendruscolo, M. Hayer-Hartl, F.-U. Hartl and R. M. Vabulas: Amyloid-like Aggregates Sequester Numerous Metastable Proteins with Essential Cellular Functions. Cell, January 7, 2011
Contact:
Prof. Dr. F.-Ulrich Hartl
Cellular Biochemistry
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
Germany
E-Mail: uhartl@biochem.mpg.de
Anja Konschak
Public Relations
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
Germany
Tel. ++49 89-8578-2824
E-Mail: konschak@biochem.mpg.de
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Making diamonds at ambient pressure
Scientists develop novel liquid metal alloy system to synthesize diamond under moderate conditions. Did you know that 99% of synthetic diamonds are currently produced using high-pressure and high-temperature (HPHT) methods?[2]…

Eruption of mega-magnetic star lights up nearby galaxy
Thanks to ESA satellites, an international team including UNIGE researchers has detected a giant eruption coming from a magnetar, an extremely magnetic neutron star. While ESA’s satellite INTEGRAL was observing…

Solving the riddle of the sphingolipids in coronary artery disease
Weill Cornell Medicine investigators have uncovered a way to unleash in blood vessels the protective effects of a type of fat-related molecule known as a sphingolipid, suggesting a promising new…





















