Chemical synthesis: A simple technique for highly functionalized compounds
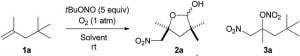
Oxidative nitration of alkene 1a to produce ɣ-lactol (2a) and nitrate ester (3a)<br>
This research is also described in the inaugural June issue of the Kanazawa University Research Bulletin: http://www.kanazawa-u.ac.jp/research_bulletin/index.html
The addition of functional groups to certain unsaturated hydrocarbons, known as alkenes, is a crucial stage in the synthesis of various compounds, including many plastics.
For these functionalization reactions to occur a carbon-hydrogen (C-H) bond must be activated, which is traditionally achieved using transition metal catalysts. However use of these catalysts has both economical and environmental drawbacks. Now researchers at Kanazawa University have demonstrated a technique that allows direct functionalization of alkenes without the need for metallic reagents, photolysis or extreme reaction conditions.
Tsuyoshi Taniguchi and colleagues at Kanazawa University developed work where they had reported a reaction of alkenes using tert-butyl nitrite and molecular oxygen. They monitored the reaction products — ɣ-lactol and nitrate ester — using different solvents, and found that a high polarity aprotic (hydrogen-free) solvent gave the best yield, with ɣ-lactol as the major product.
They then experimented with different alkenes and observed how the products differed for branched and linear alkenes. Further reduction reactions demonstrated how the new synthesis technique could yield a range of useful derivatives, producing highly functionalized compounds from simple alkenes in only one or two steps.
The researchers were also able to propose a possible reaction mechanism. While the exact pathway remains uncertain, they suggest that the key step is the cleavage of an oxygen-oxygen bond to form a highly reactive alkoxy radical – a molecular component comprising an oxygen with single bonds either side to hydrocarbon chains.
The work demonstrates how substantial yields of highly functionalized compounds can be achieved from simple organic molecules in simple conditions with no metal catalyst. The authors conclude, “We believe that such ‘simple and advanced reactions’ are promising in the development of useful synthetic methods involving direct C–H functionalization.”
Further information
Organization of Frontier Science and Innovation
Kanazawa University
Kakuma, Kanazawa, Ishikawa 920-1192, Japan
E-mail: fsojimu@adm.kanazawa-u.ac.jp
Website: http://www.o-fsi.kanazawa-u.ac.jp/en/about/
About Kanazawa University
As the leading comprehensive university on the Sea of Japan coast, Kanazawa University has contributed greatly to higher education and academic research in Japan since it was founded in 1949. The University has three colleges and 16 schools offering courses in subjects that include medicine, computer engineering, and humanities.
The University is located on the coast of the Sea of Japan in Kanazawa—a city rich in history and culture. The city of Kanazawa has cultivated a highly respected intellectual profile since the time of the Kaga fiefdom (1598–1867). Kanazawa University is divided into two main campuses: Kakuma and Takaramachi for its approximately 12,200 students including 500 from overseas.
Journal information
Publication and Affiliation
Tsuyoshi Taniguchi,* Yuki Sugiura, Takashi Hatta, Atsushi Yajima and Hiroyuki Ishibashi Multifunctionalization of alkenes via aerobic oxynitration and sp3 C–H oxidation. Chem. Commun. 49 (2013) 2198-2200
* School of Pharmaceutical Sciences, Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University, Kakuma-machi, Kanazawa 920-1192, Japan.
*corresponding author, e-mail address: tsuyoshi@p.kanazawa-u.ac.jp
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Bringing bio-inspired robots to life
Nebraska researcher Eric Markvicka gets NSF CAREER Award to pursue manufacture of novel materials for soft robotics and stretchable electronics. Engineers are increasingly eager to develop robots that mimic the…

Bella moths use poison to attract mates
Scientists are closer to finding out how. Pyrrolizidine alkaloids are as bitter and toxic as they are hard to pronounce. They’re produced by several different types of plants and are…

AI tool creates ‘synthetic’ images of cells
…for enhanced microscopy analysis. Observing individual cells through microscopes can reveal a range of important cell biological phenomena that frequently play a role in human diseases, but the process of…





















