Cellular team players
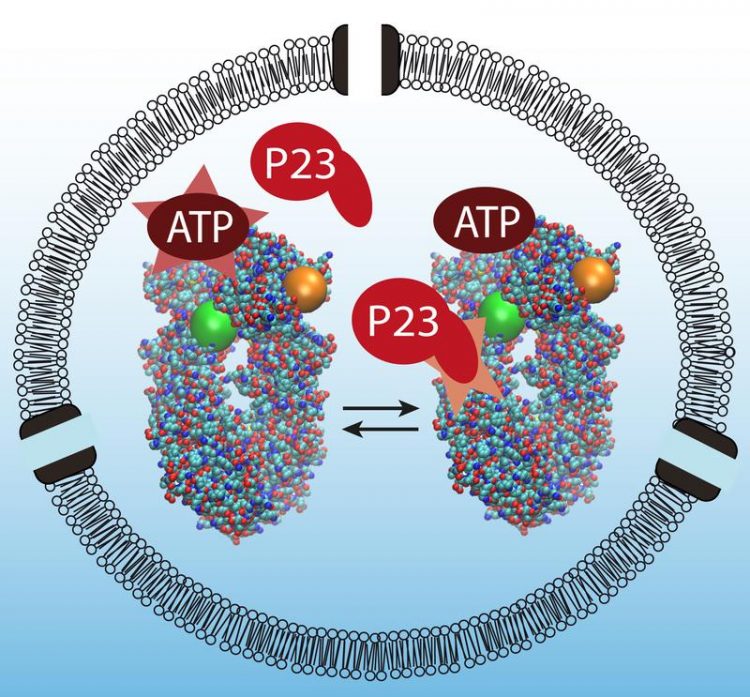
Interaction of Hsp90 with P23 - Image: Bjoern Hellenkamp / TUM
Many enzymes work only with a co-trainer, of sorts. Scientists at the Technische Universitaet Muenchen (TUM) and the Cluster of Excellence Nanosystems Initiative Munich (NIM) show what this kind of cooperation looks like in detail using a novel methodology applied to the heat shock protein Hsp90.
As in a successful football match, all actors in a cell must play in perfect coordination. A typical example for this kind of cooperation can be seen in the heat shock protein Hsp90, which controls the proper folding of other proteins. Together with a second molecule, the co-chaperone P23, it splits the energy source ATP to yield the energy it needs to do its work.
However, while normal enzyme reactions often are easy to follow because the involved proteins alter their conformations clearly, the interaction between P23 and ATP involves significantly less conspicuous changes in state.
Using a sophisticated methodology, a team led by Professor Thorsten Hugel, head of the Research Group for Molecular Machines at the TU München and member of the Cluster of Excellence Nanosystems Initiative Munich (NIM), has now managed to observe this reaction in detail for the first time – step for step with single molecules of Hsp90, P23 and ATP.
Live transmission of molecular processes
To this end, the team adapted the so-called FRET (Foerster resonance energy transfer) methodology to suit their requirements. The approach works by using a variety of fluorescent dye molecules bonded to specific sites in the involved components. When these complexes are excited with light of a specific wavelength, the pigments start to fluoresce in a kind of chain reaction. The emitted fluorescent light reveals the precise distance between the marked sites, right down to the nanometer.
To determine exactly how the components Hsp90, P23 and ATP interact with each other, the biophysicists observed the positions and bonding sequences of the individual molecules over a span of several minutes. From the resulting data they could deduce even the smallest of changes, as well as the biological function of the overall complex.
Energy production only as a team
Using this approach, the Munich researchers successfully demonstrated in detail that the P23 protein strengthens ATP bonding, thereby significantly increasing the amount of energy exploited. They also showed that the two substances bond with Hsp90 this effectively only as a team, thereby allowing ATP splitting to be used so successful.
“Without P23 the heat shock enzyme effectively runs on idle,” explains Bjoern Hellenkamp the results. “When P23 joins the game, it is like shifting into gear. The energy is released and the reaction moves clearly in one direction. This is referred to as directionality.”
In the near future the biophysicists want to investigate in detail how Hsp90 uses the exploited energy. The newly established methodology also allows them to investigate other multicomponent systems with mechanisms that have eluded study because of their minimal conformational alterations.
Publication:
Four-colour FRET reveals directionality in the Hsp90 multicomponent machinery
C. Ratzke, B. Hellenkamp, T. Hugel
Nature Communications 5, Article number: 4192. Published online: 20 June 2014
DOI: 10.1038/ncomms5192
Contact:
Prof. Dr. Thorsten Hugel
Technische Universitaet Muenchen (TUM)
Physik-Department E40
James Franck-Str. 1, 85748 Garching, Germany
E-mail – Telefon: +49 89 289 16781 – Internet
Media Contact
More Information:
http://www.tum.de/en/about-tum/news/press-releases/short/article/31639/All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















