Bad proteins branch out
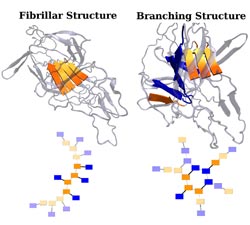
Two types of aggregate structures found in new work by researchers at Rice University are shown in three-dimensional (top) and simplified two-dimensional (bottom) representations. In the 2-D model, bold colors indicate the actual structures found in the AWSEM molecular dynamics simulations and the light colors are examples of how these structures might further develop in the presence of more protein copies. In each protein, there are two sticky segments, shown in orange and blue. A solid line represents the rest of each protein. Dashed lines represent stabilizing interactions formed between two sticky segments from different proteins. A fibrillar structure is shown on the left and a branching structure is shown on the right. The presence of two or more sticky segments in one protein allows for a greater diversity of possible aggregate structures. This realization should spur protein scientists to design experiments to investigate these different types of structures and their potential role in misfolding-related diseases. (Credit: Weihua Zheng & Nick Schafer/Rice University)<br>
A method by Rice University researchers to model the way proteins fold – and sometimes misfold – has revealed branching behavior that may have implications for Alzheimer’s and other aggregation diseases.
Results from the research will appear online this week in the Proceedings of the National Academy of Sciences.
In an earlier study of the muscle protein titin, Rice chemist Peter Wolynes and his colleagues analyzed the likelihood of misfolding in proteins, in which domains – discrete sections of a protein with independent folding characteristics – become entangled with like sequences on nearby chains. They found the resulting molecular complexes called “dimers” were often unable to perform their functions and could become part of amyloid fibers.
This time, Wolynes and his co-authors, Rice postdoctoral researcher Weihua Zheng and graduate student Nicholas Schafer, modeled constructs containing two, three or four identical titin domains. They discovered that rather than creating the linear connections others had studied in detail, these proteins aggregated by branching; the proteins created structures that cross-linked with neighboring proteins and formed gel-like networks that resemble those that imbue spider silk with its remarkable flexibility and strength.
“We’re asking with this investigation, What happens after that first sticky contact forms?” Wolynes said. “What happens if we add more sticky molecules? Does it continue to build up further structure out of that first contact?
“It turned out this protein we’ve been investigating has two amyloidogenic segments that allow for branch structures. That was a surprise,” he said.
The researchers used their AWSEM (Associative memory, Water-mediated Structure and Energy Model) program to analyze how computer models of muscle proteins interact with each other, particularly in various temperatures that determine when a protein is likely to fold or unfold.
The program relies on Wolynes’ groundbreaking principle of minimal frustration to determine how the energy associated with amino acids, bead-like elements in a monomer chain, determines their interactions with their neighbors as the chain folds into a useful protein.
Proteins usually fold and unfold many times as they carry out their tasks, and each cycle is an opportunity for it to misfold. When that happens, the body generally destroys and discards the useless protein. But when that process fails, misfolded proteins can form the gummy amyloid plaques often found in the brains of Alzheimer’s patients.
The titin proteins the Rice team chose to study are not implicated in disease but have been well-characterized by experimentalists; this gives the researchers a solid basis for comparison.
“In the real muscle protein, each domain is identical in structure but different in sequence to avoid this misfolding phenomenon,” Wolynes said. So experimentalists studying two-domain constructs made the domains identical in every way to look for the misfolding behavior that was confirmed by Rice’s earlier calculations. That prompted Wolynes and his team to create additional protein models with three and four identical domains.
“The experiments yield coarse-grained information and don’t directly reveal detail at the molecular level,” Schafer said. “So we design simulations that allow us to propose candidate misfolded structures. This is an example of how molecular models can be useful for investigating the very early stages of aggregation that are hard to see in experiments, and might be the stages that are the most medically relevant.”
“We want to get the message across that this is a possible scenario for misfolding or aggregation cases — that this branching does exist,” Zheng added. “We want experimentalists to know this is something they should be looking for.”
Wolynes said the lab’s next task is to model proteins that are associated with specific diseases to see what might be happening at the start of aggregation. “We have to investigate a wider variety of structures,” he said. “We have no new evidence these branching structures are pathogenic, but they’re clearly an example of something that happens that has been ignored until now.
“I think this opens up new possibilities in what kind of structures we should be looking at,” he said.
The National Institute of General Medical Sciences, one of the National Institutes of Health, and the D.R. Bullard-Welch Chair at Rice University supported the research. Wolynes is the Bullard-Welch Foundation Professor of Science and a professor of chemistry and a senior scientist with the Center for Theoretical Biological Physics at Rice. The researchers utilized the Data Analysis and Visualization Cyberinfrastructure (DAVinCI) supercomputer supported by the National Science Foundation and administered by Rice’s Ken Kennedy Institute for Information Technology.
Read the abstract at http://www.pnas.org/cgi/doi/10.1073/pnas.1320483110
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,708 undergraduates and 2,374 graduate students, Rice’s undergraduate student-to-faculty ratio is 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice has been ranked No. 1 for best quality of life multiple times by the Princeton Review and No. 2 for “best value” among private universities by Kiplinger’s Personal Finance. To read “What they’re saying about Rice,” go to http://tinyurl.com/AboutRiceU.
Media Contact
More Information:
http://www.rice.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















