Bacterial Roundabouts Determine Cell Shape
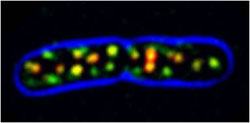
Bacillus subtilis cell with several patches of Mbl (MreB-like protein) fused to the green fluorescent protein. Colors represent overlay of images taken by total internal reflection fluorescence microscopy (TIRFM, red), epifluorescence (green) and transmitted light (blue, indicates cell outline). Picture: Roland Wedlich-Söldner / Copyright: MPI of Biochemistry<br>
As scientists at the Max Planck Institute for Biochemistry and at the French INRA now show, MreB molecules assemble into larger units, but not – as previously believed – into continuous helical structures. The circular movement of these units along the inside of the bacterial envelope is mediated by cell wall synthesis, which in turn requires the support of MreB. This mutual interaction may be a widespread phenomenon among bacteria and opens up new avenues for therapeutic intervention. The bacterial cell wall is already a major target for antibiotics.
Even single cells have to maintain their shape: In higher organisms, the supporting structures of the cytoskeleton, which include filament networks made of the protein actin, take care of this job. The much smaller bacterial cells possess similar cytoskeletal structures, such as the actin related protein MreB. Up to now, scientists believed that this molecule forms spiral structures on the inside of the cell membrane in non-spherical bacteria, which serve as a scaffold for the assembly of the comparatively rigid cell wall.
Using innovative imaging technologies based as fluorescent microscopy, the scientists in the laboratory of Roland Wedlich-Söldner now have been able to show, that MreB proteins do not form such highly ordered structures – but yet are organized in more complex ways than they had previously assumed. “MreB molecules assemble into larger units, or patches. They move in circular paths along the inside of the cell membrane, but without following a preferred direction”, explains Julia Domínguez-Escobar, PhD student at the Max Planck Institute of Biochemistry.
A highly unexpected finding of the study was that the movement of MreB patches relies on a functioning cell wall. MreB structures cannot move on their own but are pulled along the bacterial envelope by the newly synthesized cell wall material. The MreB patches are located at the inside, the cell wall at the outside of the cell membrane. Thus, interaction is likely mediated by molecules that span the cell membrane. These molecular adapters link the incorporation of newly synthesized cellular material with the MreB units, which thereby follow the permanently growing cell wall structures.
Many parts of the cell wall are almost universally conserved in bacteria, making it likely that the newly discovered mechanism is widespread. Hence, the results could play an important role for the further investigation of bacterial cells, but also for medicine: “Cell wall synthesis already is a key target for antibiotics. New insights into the structure of the cell wall could open up urgently needed therapeutic alternatives”, hopes Wedlich-Söldner. [UD]
Original Publication:
Julia Domínguez-Escobar, Arnaud Chastanet, Alvaro H. Crevenna, Vincent Fromion, Roland Wedlich-Söldner* and Rut Carballido-López* (2011): Processive movement of MreB-associated cell wall biosynthetic complexes in bacteria. Science, June 3, 2011.
* Equal contribution
Contact:
Dr. Roland Wedlich-Söldner
Cellular Dynamics and Cell Patterning
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
E-Mail: wedlich@biochem.mpg.de
Dr. Rut Carballido-López
UMR1319 Micalis
Bat. Biotechnology (440)
I.N.R.A., Domaine de Vilvert
78352 Jouy-en-Josas Cedex, France
E-Mail: rut.carballido-lopez@jouy.inra.fr
Anja Konschak
Public Relations
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
Phone +49 89 8578-2824
E-Mail: konschak@biochem.mpg.de
Media Contact
More Information:
http://www.biochem.mpg.deAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















