How bacteria integrate autotransporters into their outer membrane
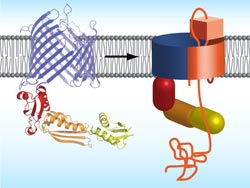
Proposed mechanism how bacteria integrate autotransporter into their outer membrane. Left: protein structure of TamA, right: TamA with autotransporters (orange).<br>
Professors Timm Maier and Sebastian Hiller from the Biozentrum of the University of Basel now demonstrate how these transporter proteins are integrated into the outer membrane.
Using x-ray structural analysis they reveal the structure-function relationship of the protein TamA, which plays an important role in the assembly of transport proteins in the bacterial outer membrane. Their findings have been published recently in the renowned scientific journal «Nature Structural and Molecular Biology».
Shuttling proteins from inside the cell to the outside environment is a complex task for Gram-negative bacteria, which are not only surrounded by an inner membrane, but also by an outer membrane barrier for protection against adverse environmental conditions.
The bacteria however, can overcome this additional barrier by inserting special transport proteins into the protective outer membrane. In a joint project, Maier and Hiller, both Professors of Structural Biology at the Biozentrum of the University of Basel, provide mechanistic insights into this key process.
The structure of the assembly protein TamA explains its function
An important option for channeling protein domains across the outer membrane are so-called autotransporters. These membrane proteins form a barrel-like structure with a central pore, but they cannot autonomously transport their “passenger domain” across the outer membrane.
Specific assembly proteins are required for the folding and integration of autotransporters into the outer membrane. Employing x-ray crystallography, the authors of the study decoded the atomic structure of the autotransporter assembly protein TamA of the intestinal bacterium Escherichia Coli.
“The protein TamA”, explains Fabian Gruss, first author and recipient of a Werner-Siemens PhD fellowship, “also forms a barrel with a pore. The pore is closed to the outside by a lid but a particular kink in the barrel wall provides a gate for autotransporter substrates.” When an unfolded autotransporter is delivered, TamA hooks onto one end of the substrate polypeptide chain and integrates it step by step via the gate into its own barrel structure.
The TamA barrel is thus expanded; the pore widens and opens such that passenger substrates traverse to the exterior. The assembly process ends when TamA releases the autotransporter into the surrounding membrane. “The autotransporter insertion mechanism was previously completely enigmatic – for the first time, knowing the structure of TamA, we can now picture how assembly and translocation could function.”
Assembly process important for infections
Many pathogens, such as the diarrhea causing Yersinia, Salmonella or the Cholera pathogen, belong to the group of Gram-negative bacteria. With the help of the autotransporter, they release toxins or adhesive proteins to infect their host cells. In their study, Maier and Hiller provide completely new findings about membrane insertion of autotransporters as well as the translocation of their cargo.
Original Citation
Fabian Gruss, Franziska Zähringer, Roman P. Jakob, Björn M. Burmann, Sebastian Hiller, Timm Maier.
The structural basis of autotransporter translocation by TamA.
Nature Structural and Molecular Biology, Published online 23 September 2013
Further Information
Prof. Dr. Timm Maier, Biozentrum of the University of Basel, Tel.: +41 61 267 21 76,
E-Mail: timm.maier@unibas.ch
Prof. Dr. Sebastian Hiller, Biozentrum of the University of Basel, Tel.: +41 61 267 20 82, E-Mail: sebastian.hiller@unibas.ch
Weitere Informationen:
http://www.nature.com/nsmb/journal/vaop/ncurrent/abs/nsmb.2689.html – Abstract
Media Contact
More Information:
http://www.unibas.chAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















