How bacteria change movement direction in response to oxygen: Molecular interactions unravelled
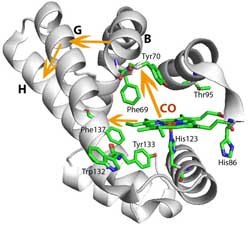
Conformational changes within HemAT: When oxygen binds to the sensor domain (for methodological reasons, the experiment was carried out with carbon monoxide, CO, instead of oxygen), the protein conformation in the vicinity of the sensor domain changes. Thus, helices B and G are displaced. This affects the neighbouring H-helix which is continuous with the signalling domain.<br>Illustration: Samir El-Mashtoly<br>
Together with colleagues from Japan, Dr. Samir El-Mashtoly from the RUB Department of Biophysics, led by Prof. Dr. Klaus Gerwert, has gained new insights into the molecular interactions during aerotaxis of Bacillus subtilis, i.e., the dependence of the movement direction on the oxygen concentration in the environment.
The research team investigated the conformational changes within the protein HemAT. Via a signal transduction chain, this protein sends a command to the flagellar motor which controls the movement direction. They report in the Journal of Biological Chemistry.
How bacteria change movement direction in response to oxygen
Molecular interactions unravelled
RUB researcher and Japanese colleagues report in the Journal of Biological Chemistry
How single cell organisms like bacteria manage to react to their environment is not yet completely understood. Together with colleagues from Japan, Dr. Samir El-Mashtoly from the RUB Department of Biophysics, led by Prof. Dr. Klaus Gerwert, has gained new insights into the molecular interactions during aerotaxis of Bacillus subtilis, i.e., the dependence of the movement direction on the oxygen concentration in the environment. The research team investigated the conformational changes within the protein HemAT. Via a signal transduction chain, this protein sends a command to the flagellar motor which controls the movement direction. They report in the Journal of Biological Chemistry.
Signal transduction chain
The signal transduction chain starts with binding of oxygen to HemAT’s heme domain, which is also known from haemoglobin in the red blood cells and is called the sensor domain of HemAT. Oxygen binding leads to a conformational change in the sensor domain. This in turn provokes several further conformational changes within HemAT that finally affect the signalling domain of the protein. The signalling domain then transmits the information about a rise in oxygen concentration to other proteins within the cell. These proteins forward the message to the motor of the flagellum. The research team investigated how the information travels from the sensor domain of HemAT to its signalling domain.
Protein helices forward the information
For that purpose, Dr. El-Mashtoly used the time-resolved ultraviolet resonance Raman spectroscopic facilities in the Picobiology Institute in Japan. This method provides, for instance, structural information about the conformation of the protein and hydrogen bonding interactions on a nanosecond to microsecond time scale. The results suggest that the conformational change in the sensor domain, i.e., the heme structure, induces the displacement of two protein helices within HemAT. This displacement affects another helix which is continuous with the structure of the signalling domain. Due to a series of conformational changes, the information about oxygen binding thus reaches the signalling domain of the protein.
Bibliographic record
S. El-Mashtoly, M. Kubo, Y. Gu, H. Sawai, S. Nakashima, T. Ogura, S. Aono, T. Kitagawa (2012): Site-specific protein dynamics in communication pathway from sensor to signaling domain of oxygen sensor protein, HemAT-Bs, Journal of Biological Chemistry, doi: 10.1074/jbc.M112.357855
Further information
Dr. Samir El-Mashtoly, Department of Biophysics, Faculty of Biology and Biotechnology at the Ruhr-Universität, 44780 Bochum, Germany, Tel. +49/234/32-29833
samir.elmashtoly@bph.rub.de
Biophysics at RUB
http://www.bph.rub.de/index_en.htm
Editor: Dr. Julia Weiler
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















