Atomic structure of essential circadian clock protein complex determined
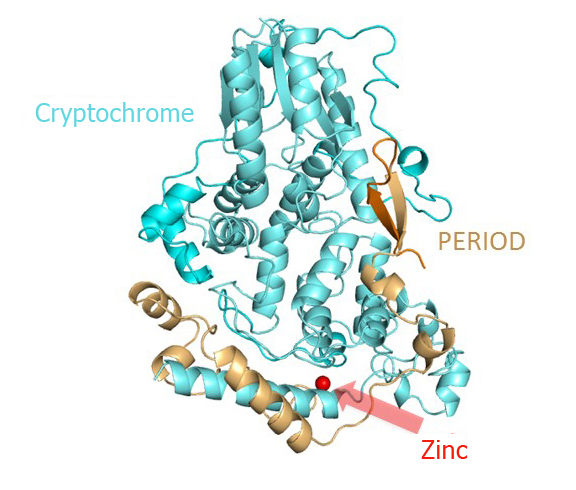
Three-dimensional structure of the mouse cryptochrome-period clock protein complex. The complex is stabilized by a zinc atom coordinated by both proteins. source: Eva Wolf, JGU
Structural biologists have made important progress towards better understanding the functioning of the circadian clock. The circadian or inner clock coordinates the sleep-wake rhythm and many other body processes that regulate, for example, metabolism, blood pressure, and the immune system.
A research team led by Professor Eva Wolf, recently appointed Professor of Structural Biology at the Institute of General Botany of Johannes Gutenberg University Mainz (JGU) and Adjunct Director at the Institute of Molecular Biology (IMB), has for the first time identified the molecular structure of a protein complex that plays an important role in regulating the circadian rhythm. At the same time, they also made a surprising discovery: The protein complex contains a zinc ion, which apparently stabilizes it. These results could form the basis for new strategies for treating illnesses that are the result of circadian clock dysfunction.
“Our circadian clock controls many important physiological functions,” explained Professor Eva Wolf. If the natural rhythm is disrupted, as for example in the case of people on shift work, the likelihood of developing metabolic disorders, diabetes, or cancer is significantly increased. The fundamental research conducted in the Wolf group is focused on obtaining insight into the molecular mechanisms of the circadian clock.. Among the currently investigated topics are the cryptochromes, a class of proteins associated with the circadian clock in mammals. In addition to regulating circadian rhythm, these also control glucose homeostasis and blood sugar levels. Together with another clock protein called period they form a complex, the structure of which has just been determined by Wolf's team.
By x-ray analysis of the cryptochrome-period complex structure, the researchers were able to observe atomic details of the interaction between the cryptochrome and period proteins and also discovered that the zinc ion mediates this interaction. “The metal ion stabilizes the complex and also appears to influence an adjacent disulfide bond,” clarified Wolf. Cell biological studies conducted in the collaborating group of Prof. Dr. Achim Kramer at the Charite Berlin showed that this also is the case in human cells.”
The researchers had not expected to detect a disulfide bond in the presence of the redox state that prevails in the cytoplasm and the cell nucleus. Its existence is probably regulated by the zinc ion and the disulfide bond itself is perhaps a sensor that indicates the metabolic status of the cell.
“We assume that the formation of this cryptochrome-period protein complex provides a mechanism by which the circadian clock interacts with the metabolism, while the zinc ion and the disulfide bond play an important role in regulating the stability of the complex,” summarized Wolf. The now Mainz-based biologist hopes that further findings about the basic functioning of the cryptochrome-period complex and her aim of determining the interaction patterns of further clock proteins may help in the development of future medical treatments.
About the Institute for Molecular Biology gGmbH
The Institute of Molecular Biology gGmbH (IMB) is a center of excellence in the life sciences that was established in 2011. Research at IMB concentrates on three cutting-edge areas: epigenetics, developmental biology, and DNA repair. The institute is a prime example of a successful collaboration between public authorities and a private foundation. The Boehringer Ingelheim Foundation has dedicated EUR 100 million for a period of 10 years to cover the operating costs for research at IMB, while the state of Rhineland-Palatinate provided approximately EUR 50 million for the construction of a state-of-the-art building.
For more information about IMB, please visit www.imb.de
About the Boehringer Ingelheim Foundation
The Boehringer Ingelheim Foundation is an independent, non-profit organization committed to the promotion of the medical, biological, chemical, and pharmaceutical sciences. It was established in 1977 by Hubertus Liebrecht (1931-1991), a member of the shareholder family of the company Boehringer Ingelheim. Through its PLUS 3 Perspectives Program and Exploration Grants, the foundation supports independent group leaders; it also endows the internationally renowned Heinrich Wieland Prize as well as awards for up-and-coming scientists. The foundation has granted EUR 100 million over a period of ten years to finance the scientific activities of the Institute of Molecular Biology (IMB).
For more information about the foundation and its programs, please visit www.boehringer-ingelheim-stiftung.de
Image:
http://www.uni-mainz.de/bilder_presse/10_botany_circadian_clock.jpg
Three-dimensional structure of the mouse cryptochrome-period clock protein complex. The complex is stabilized by a zinc atom coordinated by both proteins.
source: Eva Wolf, JGU
Publication:
Ira Schmalen et al.
Interaction of Circadian Clock Proteins CRY1 and PER2 Is Modulated by Zinc Binding and Disulfide Bond Formation
Cell 157:5, pp 1203-1215, 22 May 2014
DOI: 10.1016/j.cell.2014.03.057
Further information:
Professor Dr. Eva Wolf
Institute of General Botany / Institute of Molecular Biology (IMB)
Johannes Gutenberg University Mainz (JGU)
D 55099 Mainz, GERMANY
phone +49 6131 39-21701
fax +49 6131 39-27850
e-mail: evawolf1@uni-mainz.de
http://iabserv.biologie.uni-mainz.de/
http://www.cell.com/cell/abstract/S0092-8674(14)00535-2 ;
http://www.imb.de/wolf
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















