Atomic-Level View Provides New Insight Into Translation of Touch Into Nerve Signals
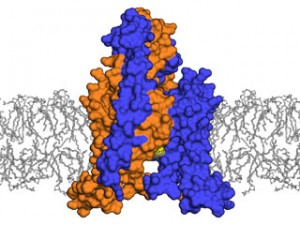
<b>Molecular roadblock:</b> The TRAAK channel (purple and orange) dampens sensations by letting potassium ions escape from a neuron. Researchers found the channel uses a never-before-seen system for blocking that flow of ions when it closes: A lipid (yellow) from the neuron membrane (gray) protrudes into the channel.
New work at Rockefeller University has revealed that one such channel in humans responds to mechanical force using a never-before-seen mechanism. Researchers led by Roderick MacKinnon, John D. Rockefeller Jr. Professor and head of the Laboratory of Molecular Neurobiology and Biophysics examined the TRAAK channel, which is involved in painful touch sensation, at the molecular and atomic levels, finding that it works by reducing the flow of potassium ions that create an electrical signal. The researchers’ findings were released yesterday (December 3) in Nature.
”It is fascinating to wonder how living cells evolved molecules capable of turning small mechanical forces, such as those associated with touch, into electrical signals in the nervous system. That question served as the impetus for this work,” MacKinnon says.
The channels that act as gates in the membranes that envelop neurons, including TRAAK, allow electrically charged atoms, called ions, to move in or out. It’s this movement that is the basis for an electrical signal that carries information.
TRAAK channels are one of 78 types of channels in the human body that transport potassium ions; there are other devoted to other ion types. By allowing potassium to trickle out of the neuron, TRAAK normally quiets the neurons, balancing out other channels, which would otherwise create a strong electrical signal for pain.
“TRAAK acts kind of like the brakes on a painful touch sensation, while other channels act as the gas. If you take away the brakes, innocuous touch becomes painful,” says first author Stephen Brohawn, a postdoc in the lab.
Prior work in the lab has shown TRAAK responds to membrane tension – that is stretching caused by a physical force. However, it wasn’t clear how this force caused the channel to open. In fact, scientists had previously only explained the workings of two mechanical-force sensing channels, both of which are found in bacteria.
After purifying the protein that makes up TRAAK, the team crystallized it and determined its structure using X-ray diffraction analysis. Based on the pattern produced by X-rays bounced off the crystallized protein, scientists can infer the structure of the molecule. But because it is difficult to get high-quality crystals from TRAAK, the researchers used antibodies that targeted it to create a sort of scaffold to help guide the formation of crystals.
In the structural images revealed by this work, the researchers found a unique system is responsible for holding off the flow of ions. TRAAK’s central cavity, through which the ions must pass, is flanked by two spiral-shaped chains called helices. When both of these chains are kinked upward, the channel is open so potassium can leave the cell. But when one of these two chains relaxes downward, it uncovers a sort of side door into the center of the neuron membrane.
Neuron membranes, like all cell membranes, consist of two layers of molecules called lipids that have heads facing outward and greasy chains extending inward. When TRAAK’s side door is open, one of those greasy chains, called an acyl chain, pokes into TRAAK’s central cavity, blocking it so no potassium can pass. No known channel uses a mechanism like this.
“This is the first time anyone has seen, at a molecular level, how mechanical force can open a channel in animals, including humans,” Brohawn says. “When the membrane stretches, TRAAK widens, sort of like a dot on a balloon that expands as it is inflated. That wider conformation pulls the helices upward, preventing an acyl chain from blocking the channel, and so leaving it open for potassium ions.”
“The direct involvement of lipid molecules in the gating mechanism begins to explain another well-known property of TRAAK channels – that their gating is sensitive to general anesthetics and other molecules known to enter the lipid membrane where they insert themselves between its acyl chains. By doing so, it appears these anesthetics can shut down pain sensations by locking TRAAK in an open position,” MacKinnon says.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Making diamonds at ambient pressure
Scientists develop novel liquid metal alloy system to synthesize diamond under moderate conditions. Did you know that 99% of synthetic diamonds are currently produced using high-pressure and high-temperature (HPHT) methods?[2]…

Eruption of mega-magnetic star lights up nearby galaxy
Thanks to ESA satellites, an international team including UNIGE researchers has detected a giant eruption coming from a magnetar, an extremely magnetic neutron star. While ESA’s satellite INTEGRAL was observing…

Solving the riddle of the sphingolipids in coronary artery disease
Weill Cornell Medicine investigators have uncovered a way to unleash in blood vessels the protective effects of a type of fat-related molecule known as a sphingolipid, suggesting a promising new…





















