Alzheimer's: newly identified protein pathology impairs RNA splicing
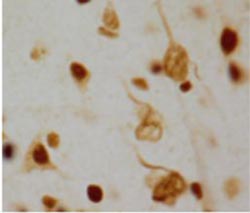
In Alzheimer's disease, all that tangles is not tau. A protein pathology identified by Emory investigators could have major implications for understanding the disease mechanism.<br><br>Tangled string-like structures involving aggregated U1 snRNP splicing proteins can be seen on the right side of this photo. The darker round structures are cell nuclei.<br>
Researchers at Emory University School of Medicine's Alzheimer's Disease Research Center have identified a previously unrecognized type of pathology in the brains of patients with Alzheimer's disease.
These tangle-like structures appear at early stages of Alzheimer's and are not found in other neurodegenerative diseases such as Parkinson's disease.
What makes these tangles distinct is that they sequester proteins involved in RNA splicing, the process by which instructional messages from genes are cut and pasted together. The researchers show that the appearance of these tangles is linked to widespread changes in RNA splicing in Alzheimer's brains compared to healthy brains.
The finding could change scientists' understanding of how the disease develops and progresses, by explaining how genes that have been linked to Alzheimer's contribute their effects, and could lead to new biomarkers, diagnostic approaches, and therapies.
The results are published in the Proceedings of the National Academy of Sciences, Early Edition.
“We were very surprised to find alterations in proteins that are responsible for RNA splicing in Alzheimer's, which could have major implications for the disease mechanism,” says Allan Levey, MD, PhD, chair of neurology at Emory University School of Medicine and director of the Emory ADRC.
“This is a brand new arena,” says James Lah, MD, PhD, associate professor of neurology at Emory University School of Medicine and director of the Cognitive Neurology program. “Many Alzheimer's investigators have looked at how the disease affects alternative splicing of individual genes. Our results suggest a global distortion of RNA processing is taking place.”
This research was led by Drs. Levey, Lah, and Junmin Peng, PhD, who was previously associate professor of genetics at Emory and is now a faculty member at St Jude Children's Research Hospital. They were aided by collaborators at University of Kentucky, Rush University, and University of Washington as well as colleagues at Emory.
Accumulations of plaques and tangles in the brains of patients with Alzheimer's disease were first observed more than a century ago. Investigating the proteins in these pathological structures has been central to the study of the disease.
Most experimental treatments for Alzheimer's have aimed at curbing beta-amyloid, an apparently toxic protein fragment that is the dominant component of amyloid plaques. Other approaches target the abnormal accumulation of the protein tau in neurofibrillary tangles. Yet the development of Alzheimer's is not solely explained by amyloid and tau pathologies, Lah says.
“Two individuals may harbor similar amounts of amyloid plaques and tau tangles in their brains, but one may be completely healthy while the other may have severe memory loss and dementia,” he says.
These discrepancies led Emory investigators to take a “back to basics” proteomics approach, cataloguing the proteins that make up insoluble deposits in the brains of Alzheimer's patients.
“The Alzheimer's field has been very focused on amyloid and tau, and we wanted to use today's proteomics technologies to take a comprehensive, unbiased approach,” Levey says.
The team identified 36 proteins that were much more abundant in the detergent-resistant deposits in brain tissue from Alzheimer's patients. This list included the usual suspects: tau and beta-amyloid. Also on the list were several “U1 snRNP” proteins, which are involved in RNA splicing.
These U1 proteins are normally seen in the nucleus of normal cells, but in Alzheimer's brains they accumulated in tangle-like structures. Accumulation of insoluble U1 protein was seen in samples from patients with mild cognitive impairment (MCI), a precursor stage to Alzheimer's, but the U1 pathology was not seen in any other brain diseases that were examined.
According to Chad Hales, MD, PhD, one of the study's lead authors, “U1 aggregation is occurring early in the disease, and U1 tangles can be seen independently of tau pathology. In some cases, we see accumulation of insoluble U1 proteins before the appearance of insoluble tau, suggesting that it is a very early event.”
For most genes, after RNA is read out from the DNA (transcription), some of the RNA must be spliced out. When brain cells accumulate clumps of U1 proteins, that could mean the process of splicing is impaired. To test this, the Emory team examined RNA from the brains of Alzheimer's patients. In comparison to RNA from healthy brains, more of the RNA from Alzheimer's brain samples was unspliced.
The finding could explain how many genes that have been linked to Alzheimer's are having their effects. In cells, U1 snRNP plays multiple roles in processing RNA including the process of alternative splicing, by which one gene can make instructions for two or more proteins.
“U1 dysfunction might produce changes in RNA processing affecting many genes or specific changes affecting a few key genes that are important in Alzheimer's,” Lah says. “Understanding the disruption of such a fundamental process will almost certainly identify new ways to understand Alzheimer's and new approaches to treating patients.”
The research was supported by the National Institute on Aging (P50AG025688, P50AG005136), the National Institute of Neurological Disorders and Stroke (P30NS055077) and the Consortium for Frontotemporal Dementia Research.
Reference: B. Bai et al. U1 Small Nuclear Ribonucleoprotein Complex and RNA Splicing Alterations in Alzheimer's Disease. PNAS Early Edition (2013).
Media Contact
More Information:
http://whsc.emory.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















