A Nanographene Hungry for Electrons
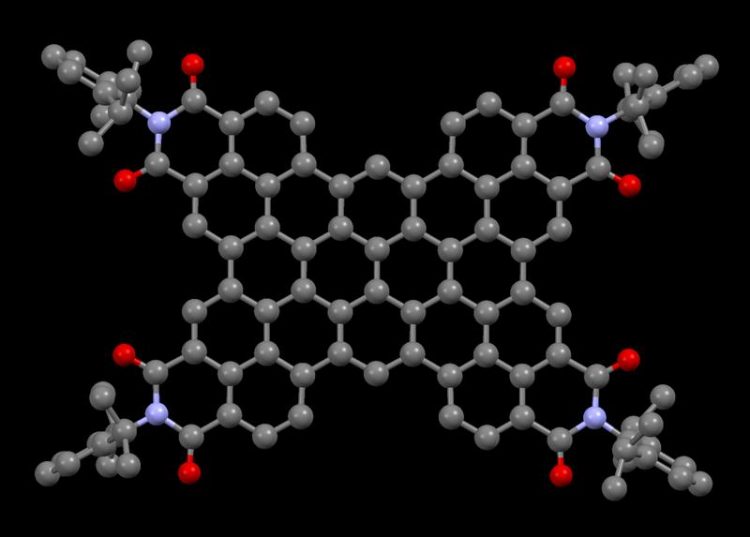
More than one nanometer in diameter is the molecule that chemists from Würzburg have synthesized for the first time. Its tendency to take up electrons makes it interesting for further investigations. Figure: Sabine Seifert
Non-specialists see a dark purple solid that appears to have no specific properties: Experts instead are delighted about a nanographene with a central core composed of 64 carbon atoms, which is characterized by its electron-poor character.
Sabine Seifert, PhD candidate at the chair of organic chemistry II, realized the synthesis and structural characterization of this molecule under supervision of Professor Frank Würthner. Recently, the journal Angewandte Chemie reported on this work in its international edition.
Capacity for up to four electrons
A pyrene precursor which is extended by four naphthalimide moieties builds up the new molecule bearing 64 sp2-hybridized carbon atoms in its central core. These coplanary arranged atoms generate a flat and two-dimensional system, which rises in dimensionality at its corners where bulky sidechains were introduced. The size of this molecule extends over more than one nanometer – one millionth of a millimeter. Its specificity: “We succeeded to synthesize one of the largest electron-poor molecules,” explains Sabine Seifert. According to the PhD student, only few similar synthetic strategies are established so far. “The synthesis in which ten carbon-carbon bonds are formed in one single chemical operation is unprecedented and may be pioneering for the fabrication of hitherto unknown polycyclic aromatic materials”, adds Professor Frank Würthner.
Electron-poor: As a consequence the new molecule has the tendency to take up additional electrons. Thus, the young junior scientist could show that up to four of them can be hosted by this system which therefore becomes interesting for organic electronics. As an organic semiconductor, it could be responsible for electron transport processes and therefore open access to new applications.
Cooperation within the Research Training School
To discover new synthetic strategies and subsequently determine the structures and properties of newly synthesized moleculesis the purpose of the cooperation of the Research Training School 2112, which was initiated at the University of Würzburg last fall with Professor Ingo Fischer as its speaker (Institute of Physical Chemistry). The focus of this program is on so called biradicals – molecules with two unpaired electrons – to which this new nanographen-symstem closely resembles once it has been charged with “only” two electrons. The generation of tri- and tetra-radicals should also be possible and therefore go beyond the aim of the Research Training School.
“We are studying how far the electrons interact with each other, how the spins behave and whether (bi)-radicaloid states can be generated”, explains Sabine Seifert. Among others, biradicals play an important role in combustion processes or atmospheric chemistry, with oxygen and ozone as well known representatives of such systems . Furthermore, their physical properties could be advantageous for the development of new optoelectronic materials. For this reason, it is the goal of the Research Training School to get an even better understanding of the respective properties and to specifically manipulate the physical and chemical characteristics of biradicals. As part of her PhD thesis, Sabine Seifert worked more than two years in the laboratory to accomplish the synthesis of this nanographene-system. The next step will be to vary the side chains and to elaborate the impact of these variations on the properties of such molecules.
An Electron-Poor C64 Nanographene by Palladium-Catalyzed Cascade C-C Bond Formation: One-Pot Synthesis and Single-Crystal Structure Analysis. Sabine Seifert, Kazutaka Shoyama, David Schmidt, and Frank Würthner. Angewandte Chemie, DOI: 10.1002/ange.201601433
Contact
Prof. Dr. Frank Würthner, Institut für Organische Chemie der Universität Würzburg
T: (0931) 31-85340, wuerthner@chemie.uni-wuerzburg.de
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















