A new model — and possible treatment — for staph bone infections
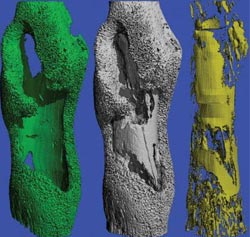
S. aureus causes profound changes in bone remodeling during infection. James Cassat, M.D., Ph.D., and colleagues developed micro-CT imaging methods to view the infected mouse femur (gray) and to quantitate and view bone formation (green) and bone destruction (yellow).<br><br>Credit: (Image courtesy of James Cassat/Vanderbilt University)<br>
Now, Vanderbilt microbiologist Eric Skaar, Ph.D., MPH, and colleagues have identified a staph-killing compound that may be an effective treatment for osteomyelitis, and they have developed a new mouse model that will be useful for testing this compound and for generating additional therapeutic strategies.
James Cassat, M.D., Ph.D., a fellow in Pediatric Infectious Diseases who is interested in improving treatments for children with bone infections, led the mouse model studies. Working with colleagues in the Vanderbilt Center for Bone Biology and the Vanderbilt University Institute of Imaging Science, Cassat developed micro-computed tomography (micro-CT) imaging technologies to visualize a surgically introduced bone infection in progress.
“The micro-CT gives excellent resolution images of the damage that's being done to the bone,” said Skaar, the Ernest W. Goodpasture Professor of Pathology. “We found that staph is not only destroying bone, but it's also promoting new bone growth. Staph is causing profound changes in bone remodeling.”
Cassat also established methods for recovering – and counting – bacteria from the infected bone.
“We're not aware of any other bone infection models where you can pull the bacteria out of a bone and count them in a highly reproducible manner,” Skaar said. “From a therapeutic development standpoint, we think this model is going to allow investigators to test new compounds for efficacy against bone infections caused by staph or any other bacteria that cause osteomyelitis.”
Several pharmaceutical companies have already approached Skaar and his team about testing compounds in the new bone infection model, which the investigators describe in the June 12 issue of Cell Host & Microbe.
Using the model, the team demonstrated that a certain protein secreted by staph plays a critical role in the pathogenesis of osteomyelitis. Understanding the specific bacterial factors – and the bone cell signals – that promote bone destruction and formation during infection could lead to new strategies for restoring bone balance, Skaar said.
“Even if it's not possible to kill the bacteria, compounds that manipulate bone growth or destruction might have some therapeutic benefit.”
Still, Skaar is interested in treatments that will eliminate the infection.
The staph bacteria involved in osteomyelitis and in other persistent infections (such as lung infections in cystic fibrosis) are often a sub-class of staph known as “small colony variants.” These staph variants grow slowly and are resistant to entire classes of antibiotics commonly used to treat bone and lung infections, Skaar said.
One way that staph bacteria become antibiotic-resistant small colony variants is by changing the way they generate energy. Instead of using respiration, they switch to fermentation, which blocks antibiotic entry and slows bacterial growth.
In a high-throughput screen for compounds that activate a heme-sensing bacterial pathway, graduate student Laura Mike identified a compound that kills fermenting staph. The findings are reported in the May 14 issue of the Proceedings of the National Academy of Sciences.
“This is a completely new molecular activity,” Skaar said. “We don't know of other molecules that are toxic against fermenting bacteria.”
The compound – and derivatives synthesized by Gary Sulikowski, Ph.D., and his team – might be useful in treating staph small colony variants, or in preventing their emergence.
The investigators demonstrated in culture that treating staph with the antibiotic gentamicin forced it to become a small colony variant and ferment, and that co-treatment with the new compound prevented resistance and killed all of the bacteria.
“We think a really interesting therapeutic strategy for this compound is that it might augment the antimicrobial activity of existing classes of antibiotics by preventing resistance to them – it might extend the lifetime of these classes of antibiotics,” Skaar said.
This would be similar to the drug Augmentin, which combines a traditional penicillin-type antibiotic and a compound that blocks bacterial resistance.
The investigators are excited to test the new compound in the mouse model of osteomyelitis. First, they will treat the mice with gentamicin and assess whether staph small colony variants form. If so, they will co-administer the new compound to test if it prevents resistance, and they will also assess it as a single treatment for the persistent infection.
Skaar stressed that Vanderbilt's collaborative environment made these studies possible. Daniel Perrien, Ph.D., and Florent Elefteriou, Ph.D., in the Vanderbilt Center for Bone Biology and colleagues in the Vanderbilt University Institute of Imaging Science were critical in facilitating development of the bone infection model. Sulikowski and other colleagues in the Vanderbilt Institute of Chemical Biology (VICB) enabled the compound development.
“This is exactly the kind of work the VICB is promoting – getting biologists like me together with chemists, to make new therapeutics,” Skaar said.
The research was supported by the Searle Scholars Program and grants from the National Institutes of Health (AI069233, AI073843, RR027631, AI091856, HD060554), including the Southeastern Regional Center of Excellence for Emerging Infections and Biodefense (AI057157).
Media Contact
More Information:
http://www.vanderbilt.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















