'Humanized' mice developed at OHSU enable malaria research breakthrough at Seattle BioMed
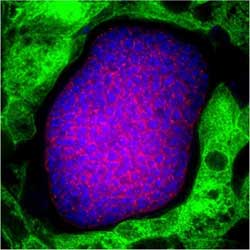
This image shows a maturing Plasmodium falciparum liver stage parasite in the liver of the human liver-chimeric FRGTM KO. Membranes of the developing merozoites are shown in red, DNA in blue, and the human hepatocytes within the liver-chimeric FRGTM KO mouse are shown in green.<br><br>Credit: Seattle Biomedical Research Institute<br>
A novel human liver-chimeric mouse model developed at Oregon Health & Science University and Yecuris Corporation has made possible a research breakthrough at Seattle Biomedical Research Institute that will greatly accelerate studies of the most lethal forms of human malaria.
The study findings are published online in the Journal of Clinical Investigation. Study photos were selected to appear in “Scientific Show Stoppers” on the JCI blog.
Plasmodium falciparum, one of two human-specific malaria parasites, is a global health crisis, causing more than 216 million new infections annually and resulting in an estimated 655,000 deaths, according to the World Health Organization.
Sporozoites, the infectious form of the parasite, are spread to people through the bites of infected mosquitos and multiply in the human liver during the initial stages of infection. There, they undergo liver stage development, culminating in the formation and release of tens of thousands of merozoites, the parasitic phase of development that infects red blood cells.
Until now, there have been few data on human malaria liver stage biology due to the lack of a viable small animal model and because liver stage P. falciparum does not grow well in a dish. Consequently, most research and therapeutics to date have targeted the human blood stage of P. falciparum's development because it replicates well in culture.
The liver-to-blood stage of P. falciparum is the focus of this research because the parasite is virtually harmless, causing no disease symptoms, prior to its transition to the blood stage.
In this study, researchers at Seattle Biomedical Research Institute, Yecuris Corporation, Oregon Health & Science University and The Rockefeller University have demonstrated that a complete liver-to-blood stage infection of P. falciparum is possible using a unique immunocompromised mouse model engrafted with human liver-chimeric cells.
The mouse model, termed the FRGTM KO mouse, was developed by paper co-author and internationally accomplished stem cell researcher Markus Grompe, M.D., in the Papé Family Pediatric Research Institute, a research arm of Oregon Health & Science University Doernbecher Children's Hospital.
In 2007 the technology was licensed to Yecuris Corporation, a biotechnology company that now produces the model and human hepatocytes on a commercial scale. As a result of this work, the FRGTM KO mouse now will be used to study new drug interventions, parasite attenuation and innate immune responses to P. falciparum liver stage infection.
The scientists also report that through the infection of the FRGTM KO mouse model, they were able to observe a previously unknown expression of proteins in liver stage development in humans that may be exploited for intervention. Equally important, they say, the FRGTM KO mouse could well provide unique opportunities for the study of another severe form of human malaria, Plasmodium vivax.
“These breakthroughs are remarkable and highlight OHSU and Yecuris' contributions to local biotechnology and research breakthroughs globally. The next generation mouse model we're developing will have a human immune system that will allow us to test not just drugs, but vaccines, which has never been done for parasitic diseases,” said Grompe, Ray Hickey Chair and Director of the Papé Family Pediatric Research Institute, OHSU Doernbecher Children's Hospital; and professor of pediatrics, and molecular and medical genetics, OHSU School of Medicine.
Grompe founded Yecuris Corporation in 2007 and is a shareholder. John Bial, who joined Yecuris in 2009, is president and chief executive officer.
“The extensive collaborative relationships and risk-taking involved in planning and executing this research is a testament to the tireless dedication of these teams to solving one of the globe's oldest killers. It also highlights how private and public funding can come together effectively to address critical challenges in global health,” said Bial.
“This first demonstration of the newly developed dual humanized FRGTM KO system is a good introduction to the kinds of translational medicine benefits that we can expect to see from these technologies. We anticipate that the next frontier for these systems will be as platforms for human vaccine development and validation, which may very likely first be tested in the area of malaria,” Bial explained.
Investigators who contributed to this work include: Stefan Kappe, Ashley Vaughan, Sebastian Mikolajczak, Alexis Kaushansky, Nelly Camargo, Seattle Biomedical Research Institute; Elizabeth Wilson, John Bial, Yecuris Corporation; Markus Grompe, Papé Family Pediatric Research Institute, Oregon Stem Cell Center, Oregon Health & Science University, Doernbecher Children's Hospital; and Alexander Ploss, Center for the Study of Hepatitis C, The Rockefeller University.
Today's JCI study, “Complete Plasmodium falciparum liver stage development in liver-chimeric mice,” was funded by grants to the Seattle Biomedical Research Institute from the Bill and Melinda Gates Foundation and the Department of Defense.
The Oregon Health & Science University research leading to the development of the FRGTM KO mouse was funded by the National Institutes of Health and The Oregon Opportunity fund.
ABOUT OHSU
Oregon Health & Science University is the state's only health and research university. With more than 1,100 principal investigators working on 4,500 basic science and applied research projects, our breakthroughs lead to new cures, new standards of care and a better understanding of the basic science that drives biomedical discovery. OHSU serves patients from every corner of the state, is a conduit for learning for more than 4,000 students and trainees, and is the source of more than 200 community outreach programs that bring health and education services to each county in the state.
ABOUT YECURIS
Portland, Ore.-based Yecuris Corporation was formed in April 2007 to commercialize transgenic mouse technology that was developed in the lab of Dr. Markus Grompe at the Oregon Health & Science University. The FAH(null) / RAG2(null) / IL2RG(null) (FRG™) mice were originally developed to explore pathways for Hereditary Tyrosinemia Type 1 (HT1), an often fatal pediatric disease. Based in part on support from the Grompe lab and the Yecuris™ mouse, medications have been developed to aid in the treatment of this disease.
ABOUT SEATTLE BIOMEDICAL RESEARCH INSTITUTE
Seattle BioMed is the largest independent, non-profit organization in the U.S. focused solely on infectious disease research. Our research is the foundation for new drugs, vaccines and diagnostics that benefit those who need our help most: the 14 million who will otherwise die each year from infectious diseases, including malaria, HIV/AIDS and tuberculosis. Founded in 1976, Seattle BioMed has nearly 400 staff members. By partnering with key collaborators around the globe, we ensure that our discoveries will save lives sooner.
Media Contact
More Information:
http://www.ohsu.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















