Scientists Create New Material With Varying Densities of Gold Nanoparticles

Material could be used to make better filters, more efficient sensors, and faster catalysts
For the first time, scientists have created a material with a gradient of gold nanoparticles on a silica covered silicon surface using a molecular template. The material, which was developed at North Carolina State University (NCSU) and tested at theNational Synchrotron Light Source(NSLS) at the U.S. Department of Energy’s Brookhaven National Laboratory, provides the first evidence that nanoparticles — each about one thousand times smaller than the diameter of a human hair — can form a gradient of decreasing concentration along a surface. A description of the material appears as the cover story in the July 23 issue of Langmuir.
“This material promises to be the first in a series with many applications in electronics, chemistry, and the life sciences,” said Rajendra Bhat, a Ph.D. student from North Carolina State University (NCSU) and the lead author of the study. Bhat worked with Jan Genzer, a chemical engineering professor at NCSU, and Daniel Fischer, a physicist from the U.S. Department of Commerce’s National Institute of Standards and Technology (NIST).
 |
|
|
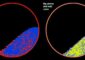
“Top: Images of gold nanoparticles attached to the silica surface at different distances from the most populated end of the substrate. As the distance increases, the number of particles decreases, revealing a particle gradient. Bottom: Simplified representation of the material showing particles in decreasing concentration along the surface. “ |
To build the material, the scientists first prepared a very thin layer of organosilanes, sticky molecules with a head and a tail, on a rectangular surface of silica. The head glues to the surface, while the tail sticks out, acting like a hook waiting for a gold nanoparticle to attach to it, explained Genzer, leader of the NCSU team. The molecules, emitted vertically in the form of a vapor by a source close to one side of the surface, slowly fell on it with decreasing concentration as the distance from the source increased, thus creating a gradient to serve as a molecular template.
The next step was to dip the material in a solution containing the gold nanoparticles, each of which was coated with a negatively charged chemical. In the solution, the tails of the organosilane molecules took on a positive charge, so the negatively charged gold particles attached to the oppositely charged tails underneath.
To visualize the gradient of gold particles, Bhat and his colleagues used an atomic force microscope, in which a tiny needle moves along the surface, following its bumps and valleys to reveal its topography. To look at the gradient of the organosilane molecules, the scientists used a technique called near-edge x-ray absorption fine structure (NEXAFS). In NEXAFS, extremely intense x-ray light is sent toward the material, and the electrons emitted by the material and collected with a sensitive detector provide information about the concentration of the organosilane molecules on the surface.
| |
“We needed to confirm that both the gold particles and the sticky groups followed the same underlying gradient template,” Bhat said. “The results from both techniques were expected to coincide if the particles were attaching to the underlying layer of sticky molecules. Our results show exactly that.”
“The distinguishing feature of our approach is that the particles follow a pre-designed chemical template provided by the organosilane sticky groups,” said Genzer. “The ability to manipulate the underlying template allows us to prepare gradient structures of nanoparticles with varying characteristics.”
| |
The main advantage of the gradient structure is that large numbers of structures can be combined on a single substrate and used for high-throughput processing. It might, for example, save time for chemists testing clusters of nanoparticles used as catalysts — chemicals actively sought by the chemical industry to create new, less polluting sources of energy. “Clusters made of different numbers of nanoparticles could be put on a single surface, and scientists could test this surface only once in a chemical reaction, instead of having to run each cluster separately through the reaction,” Fischer said. The material could also be used as a sensor to detect species that have specific affinities for nanoparticles, or as a filter to select particles of given sizes.
Bhat and his colleagues are now exploring the properties of similar materials, with different “sticky” substances and nanoparticles. “This research is so new that we are still thinking of potential applications for these materials,” he said.
This research was funded by the U.S. Department of Energy, which supports basic research in a variety of scientific fields, the National Science Foundation, and the Department of Commerce.
The U.S. Department of Energy’s Brookhaven National Laboratory conducts research in the physical, biomedical, and environmental sciences, as well as in energy technologies. Brookhaven also builds and operates major facilities available to university, industrial, and government scientists. The Laboratory is managed by Brookhaven Science Associates, a limited liability company founded by Stony Brook University and Battelle, a nonprofit applied science and technology organization. |
Media Contact
All latest news from the category: Interdisciplinary Research
News and developments from the field of interdisciplinary research.
Among other topics, you can find stimulating reports and articles related to microsystems, emotions research, futures research and stratospheric research.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…