How the newest diesel engines emit very little greenhouse gas nitrous oxide
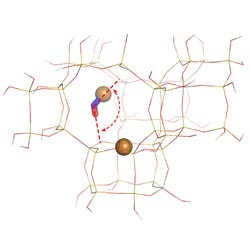
This computer model of a zeolite catalyst shows nitric oxide (ball-and-stick) interacting with a positively charged copper ion (copper ball) at an unexpected angle (red dotted lines).<br>Photo courtesy of Kwak et al.<br>
The newest catalytic converters in diesel engines blast away a pollutant from combustion with the help of ammonia. Common in European cars, the engines exhaust harmless nitrogen and water. How they do this hasn't been entirely clear. Now, new research shows that the catalyst attacks its target pollutant in an unusual way, providing insight into how to make the best catalytic converters.
Reporting in the journal Angewandte Chemie International Edition, a team of researchers in the Institute for Integrated Catalysis at the Department of Energy's Pacific Northwest National Laboratory led by chemist Janos Szanyi showed that the artificial catalyst works much the same way that similar bacterial enzymes do: by coming at the target from the side rather than head on.
“What I find exciting is the correlation between this artificial catalysis and enzyme catalysis,” said Szanyi. “Nature is telling us what to do. Nature's been at it for many millions of years, and it does this beautifully.”
Surprise Chemistry
Zeolites are crystalline alumino-silicate minerals that can accommodate metal ions — metal atoms with a slight charge — for catalytic applications. In some catalytic zeolites, the metal ions can break down the pollutant nitric oxide in vehicle emissions. However, the zeolites crumble and clog easily, leading to early failure. In addition, they produce as a byproduct the greenhouse gas nitrous oxide (known to dental patients everywhere as laughing gas).
Recently, researchers have produced a new zeolite that is surprisingly stable and makes very little nitrous oxide from nitric oxide, a chemical that depletes ozone. The zeolite produces mainly water and atmospheric nitrogen — the main component of air — but it needs to be fed ammonia, such as from urea.
Some of the diesel vehicles in Europe are now using this catalyst, and drivers must top off their urea tank as well as their diesel. Called Cu-SSZ-13, the zeolite uses copper as its added metal and has smaller spaces in its alumino-silicate scaffolding compared to other zeolites.
Researchers have assumed that this zeolite would break down nitric oxide in the same way that other zeolites do, following the same series of chemical reaction steps. However, something else must be going on because researchers can make the older zeolites work faster by adding nitrogen dioxide — but Cu-SSZ-13 doesn't respond in the same way. This indicates Cu-SSZ-13 must be taking a different chemical route.
To explore how Cu-SSZ-13 breaks down nitric oxide, the team of researchers investigated the structure of the zeolite in the process of performing the reaction. Using tools designed to find such answers at EMSL, DOE's Environmental Molecular Sciences Laboratory on PNNL's campus, team members first looked at what molecules stuck to the surface of the zeolite.
There they unexpectedly found a charged nitric oxide molecule bound to the copper ions. This molecular combination could only happen one of two ways, the more common of which requires the presence of nitrogen dioxide. Because the researchers saw no nitrogen dioxide, they ruled out that common reaction pathway.
That left the copper metal itself directly hooking up with nitric oxide. In the process, copper borrows one of nitric oxide's electrons, giving nitric oxide a charge. This early theft sets the stage for ammonia to react with the charged nitric oxide in the first of several chemical steps, ultimately pumping out atmospheric nitrogen and water.
Catalysis Imitating Life
Zooming in on the zeolite's structure and reconstructing it with NWChem, software that models molecular chemistry, the team found something unusual. In most zeolite catalysts, nitric oxide is essentially a barbell combining a nitrogen atom and an oxygen atom. The barbell is bound to the metal atom on its head, most often at the nitrogen end of the molecule. However, in Cu-SSZ-13, the copper metal bonded with both the nitrogen and the oxygen halves of the nitric oxide barbell, as if the copper and nitric oxide formed a three-membered ring. Chemists refer to this orientation as “side-on”.
“The side-on complex is uncommon in this type of synthetic catalysis,” said Szanyi. “But bacteria have an enzyme called nitrite reductase that works this way. This enzyme breaks down nitrites into atmospheric nitrogen.”
The chemists also determined that the side-on angle causes the barbell to bend slightly. With no bend, the angle is 180 degrees, but positioned within the zeolite, the angle between the nitrogen and oxygen is about 146 degrees.
The computer reconstruction of Cu-SSZ-13 in action showed the spaces within the aluminum and silicon lattice can only hold one nitric oxide molecule. Other zeolites have almost twice as much space for the nitric oxide to move around.
“The small pore size just fits the reactants and provides precise control,” said Szanyi. “This reaction mechanism explains the prior studies — things like why we don't get nitrous oxide.”
The researchers are continuing to explore whether this side-on intermediate is common in other catalyst materials and in other reactions.
This work was supported by the Department of Energy Office of Energy Efficiency and Renewable Energy.
Reference: Ja Hun Kwak, Jong H. Lee, Sarah D. Burton, Andrew S. Lipton, Charles H. F. Peden, and Janos Szanyi. A Common Intermediate for N2 Formation in Enzymes and Zeolites: Side-On Cu-Nitrosyl Complexes, Angewandte Chemie International Edition, Aug. 12, 2013, doi: 10.1002/anie.201303498.
Media Contact
More Information:
http://www.pnnl.govAll latest news from the category: Ecology, The Environment and Conservation
This complex theme deals primarily with interactions between organisms and the environmental factors that impact them, but to a greater extent between individual inanimate environmental factors.
innovations-report offers informative reports and articles on topics such as climate protection, landscape conservation, ecological systems, wildlife and nature parks and ecosystem efficiency and balance.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















