Climate change: How does soil store CO2?
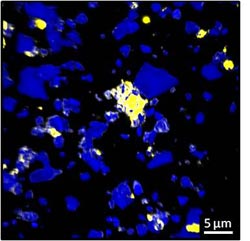
Carbon tends to bind to specific rough mineral surfaces in the soil (yellow areas). (Image: C. Vogel/TUM)<br>
Global carbon dioxide (CO2) emissions continue to rise – in 2012 alone, 35.7 billion tons of this greenhouse gas entered the atmosphere*. Some of this CO2 is absorbed by the oceans, plants and soil. As such, they provide a significant reservoir of carbon, stemming the release of CO2.
Scientists have now discovered how organic carbon is stored in soil. Basically, the carbon only binds to certain soil structures. This means that soil’s capacity to absorb CO2 needs to be re-assessed and incorporated into today’s climate models.
Previous studies have established that carbon binds to tiny mineral particles. In this latest study, published in Nature Communications, researchers of the Technische Universität München (TUM) and the Helmholtz Zentrum München have shown that the surface of the minerals plays just as important a role as their size. “The carbon binds to minerals that are just a few thousandths of a millimeter in size – and it accumulates there almost exclusively on rough and angular surfaces,” explains Prof. Ingrid Kögel-Knabner, TUM Chair of Soil Science.
The role of microorganisms in sequestering carbon
It is presumed that the rough mineral surfaces provide an attractive habitat for microbes. These convert the carbon and play a part in binding it to minerals. “We discovered veritable hot spots with a high proportion of carbon in the soil,” relates Cordula Vogel, the lead author of the study. “Furthermore, new carbon binds to areas which already have a high carbon content.”
These carbon hot spots are, however, only found on around 20 percent of the mineral surfaces. It was previously assumed that carbon is evenly distributed in the soil. “Thanks to our study, we can now pin-point the soil that is especially good for sequestering CO2,” continues Kögel-Knabner. “The next step is to include these findings in carbon cycle models.”
Mass spectrometer helps to visualize molecules
The sample material used by the team was loess, a fertile agricultural soil found in all parts of the world – which makes it a very important carbon store. The researchers were able to take ultra-precise measurements using the NanoSIMS mass spectrometer. This procedure allowed them to view and compare even the most minute soil structures.
*Source: Global Carbon Atlas
Publication:
Submicron structures provide preferential spots for carbon and nitrogen sequestration in soils, Cordula Vogel, Carsten W. Müller, Carmen Höschen, Franz Buegger, Katja Heister, Stefanie Schulz, Michael Schloter & Ingrid Kögel-Knabner, Nature Communications, DOI: 10.1038/ncomms3947.
Contact:
Prof. Dr. Ingrid Kögel-Knabner
Technische Universität München
Chair of Soil Science
Tel: +49 8161 71-3677
koegel@wzw.tum.de
Media Contact
All latest news from the category: Earth Sciences
Earth Sciences (also referred to as Geosciences), which deals with basic issues surrounding our planet, plays a vital role in the area of energy and raw materials supply.
Earth Sciences comprises subjects such as geology, geography, geological informatics, paleontology, mineralogy, petrography, crystallography, geophysics, geodesy, glaciology, cartography, photogrammetry, meteorology and seismology, early-warning systems, earthquake research and polar research.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…