Pharmaceutical track & trace solutions from Octum – safety for patients, transparency for logistics!
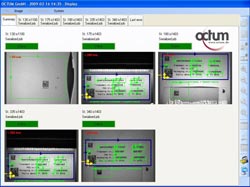
OCTUMISEr 21 CFR Part 11 user interface for track&trace solutions.
The unique marking and doubtless identification of the marking on any product, individual packaging, bundle or delivery package up to the pallet, is key for a seamless traceability over the entire logistic chain.
Different legislations regarding the identification and serialisation of pharmaceutical products comming into effect arround the world require flexible automatic identification systems for these different marking procedures in place already or comming soon. For many years Octum identication (ID) systems have a prooven track of records at machine builders and end users as an essential component of flexible track & trace solutions.
Implementation.
Octum ID systems read and verify all variable and fixed imprints on labels or direct part markings of pharmaceutical products. These ID systems provide fast and reliable OCR/OCV as well as code reading functionality for 1D- and 2D codes like pharmacode or DataMatrixCode (DMC) complying or not to GS1 standard. Imprints and markings are identified and verified in visible or invisible range like UV or IR. The ID stations may be integrated in any place of the packaging line and may perform as well other inspection tasks like symbol inspection, label position detection besides of code or character reading and verification.
Typical variable data to be inspected on labels and products are:
– Expiry date (ex. „2011-11-14“)- static data within a batch
– Manufacturing date (ex. „2009-12-10“) – static data within a batch
– Batch No. (ex. „957148“) – static data within a batch
– product type or dosis (ex. „0,4mg/L“) – static data within a batch
– Serialisation No. (z.B. „123456“) – dynamic data
Typical nonvariable symbols to be inspected for presence and integrity could be.:
– „EXP“, „LOT“, „CCYY-MM-DD“ …
The individual inspection stations on the packaging line are designed custom specific, either as networked smart cameras or PC-based systems with linescan or matrix cameras. The system software OCTUMISEr provides the ideal user interface for networked smart cameras offering a single point of interaction platform for up to 99 smart cameras. OCTUMISEr is also available as 21 CFR part 11 version with integrated audittrails and extended protocol and documentation functionality. According to his specific acces rights, the user may just choose the format to be inspected or teach new symbols and character fonts or even configure a complete new format.
The PC based sytems CV-600 with the system software CV-Inspect are also available as 21 CFR part 11 versions.
Easy integration into the existing IT infrastructure of the packaging line is ensured by the different standard hard- and software interfaces available up to direct integration into the HMI system of the line. Save archiving of data and protocols is done via the integrated standard SQL data base. Depending on the specific application and implementation, cycle times up to 900pc/min are achieved.
Customer benefit
Octum ID systems offer in a fast and reliable manner the necessary data for a seamless traceability and documentation from the individual pharmaceutical product to the final transport packaging. These ID systems are a safe investment due to their flexibility to comply with different tasks and standards as well as their easy up-grading possibilities contributing at the same time to higher patients safety.
Contact fort the Press:
Octum GmbH
Renntalstraße 16
74360 Ilsfeld
Tel. 07062 914 940
e-mail: info@octum.de
Media Contact
More Information:
http://www.octum.deAll latest news from the category: Corporate News
Newest articles

Combatting disruptive ‘noise’ in quantum communication
In a significant milestone for quantum communication technology, an experiment has demonstrated how networks can be leveraged to combat disruptive ‘noise’ in quantum communications. The international effort led by researchers…

Stretchable quantum dot display
Intrinsically stretchable quantum dot-based light-emitting diodes achieved record-breaking performance. A team of South Korean scientists led by Professor KIM Dae-Hyeong of the Center for Nanoparticle Research within the Institute for…

Internet can achieve quantum speed with light saved as sound
Researchers at the University of Copenhagen’s Niels Bohr Institute have developed a new way to create quantum memory: A small drum can store data sent with light in its sonic…