Insights gained from molecular modeling may lead to better insecticides
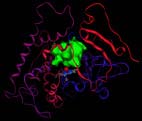
The modeled structure of the CYP6B8 protein in the corn earworm (Helicoverpa <br>zea). A potential substrate binding cavity, in green, where insecticides or plant defense chemicals can be detoxified, is shown above the heme, the small complex that includes the red sphere at its center <br>Photo courtesy of Jerome Baudry
One of the most damaging crop pests, the corn earworm, may be outwitting efforts to control it by making structural changes in a single metabolic protein, but new insights uncovered by molecular modeling could pave the way for more efficient insecticides, say researchers at the University of Illinois at Urbana-Champaign.
In a study that compared the ability of corn earworms (Helicoverpa zea) and black swallowtail butterflies (Papilio polyxenes) to neutralize insecticides and plant defense allelochemicals that target insect herbivores, researchers focused on the insectsÕ primary detoxifying cytochrome P450 enzymes.
The study was published online Monday (Feb. 23) in advance of regular publication in the Proceedings of the National Academy of Sciences.
Earworms, which can feed on hundreds of different kinds of plants, have evolved generalist counter-defense P450 proteins that can process more diverse arrays of harmful agents than can similar proteins in black swallowtails, which consume a restricted diet of only two plant families.
Predictive three-dimensional modeling of the structures of the proteins detoxifying allelochemicals and insecticides has indicated vivid differences in the catalytic sites of CYP6B1, the P450 in black swallowtails, and CYP6B8, the P450 protein in earworms.
Because the corn earwormÕs metabolic protein is more flexible, it can bind to and detoxify six different kinds of plant defense chemicals as well as three common insecticides, said Jerome Baudry, a senior research scientist in the School of Chemical Sciences at Illinois. “This generalist insect has adapted to the natural chemical defenses of plants so that it can feed on a wider variety of plants,” he said.
The P450 studied in the specialist is significantly more constrained. It contains a more rigid catalytic pocket that restricts the range of plant chemicals and insecticides that can enter and be processed, Baudry said.
While the specialization allows for much higher rates of detoxification of chemicals that black swallowtails normally encounter, they can handle few other toxins. In the study, the CYP6B1 protein metabolized only two plant defense chemicals and one insecticide.
“This is the first clear demonstration that resistance to plant allelochemicals and insecticides can be acquired by changes within a single P450 catalytic site,” said Mary A. Schuler, a professor of cell and structural biology. “If you can identify the P450 responsible for metabolizing insecticides and find a way to inactivate its catalytic site, you kill the P450 and prevent it from detoxifying insecticides.”
Accomplishing that, however, wonÕt be easy because there is at least one other P450 in corn earworms that also handles insecticides, she said. “To truly hit the earworms, you would need to find one inhibitor that can kill both enzymes. Ultimately, it may be possible to use a synergistic approach that would kill more insects using significantly lower levels of insecticides, thereby reducing the toxicity of insecticides in the environment,” she said.
Structural differences of the P450s involved in these chemical detoxifications result from changes in the arrangement of amino acids within the catalytic sites. In the black swallowtailÕs version, aromatic rings protrude into the substrate binding site, creating a rigid space in which allelochemicals or insecticides must fit exactly Ð like keys going into locks, Baudry said. The amino acid residues in the catalytic site stabilize the toxic substrate so it is optimally bonded with the proteinÕs heme, an iron-containing pigment in the catalytic site that mediates oxidation of the chemical to a non-toxic product.
In the earworm protein, many of the aromatic rings are missing, creating a much more accessible and flexible catalytic site. As a result, toxins of many different shapes and sizes can enter and be detoxified. Since the toxins are not as rigidly restricted, they are not detoxified quite as efficiently as some of the toxins encountered by the specialist P450.
“The corn earworm thus is jack of many trades but master of none, but this biochemical ability allows it to acquire new host plants and overcome new pesticides with relative ease,” said co-investigator May R. Berenbaum, the head of the entomology department at Illinois and an expert on allelochemicals.
Xianchun Li, a doctoral student in entomology, also was a coauthor of the paper and a major contributor to the research.
The study was funded by grants from the U.S. Department of Agriculture to Schuler and Berenbaum, a grant from the National Institutes of Health to Schuler, and a China Natural Science Foundation grant to Li.
Media Contact
All latest news from the category: Agricultural and Forestry Science
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















