Why spiders don't drop off of their threads
“The strength of spider dragline silk exceeds that of any material produced in laboratories, by far. All attempts to manufacture threads of similar strength have failed thus far,” explains Professor Horst Kessler, Carl von Linde Professor at the Institute for Advanced Study at the TU Muenchen (TUM-IAS).
In collaboration with the workgroup of Prof. Thomas Scheibel, who was a researcher at the TU Muenchen until 2007 and who now holds a chair of the Institute of Biomaterials at the Universitaet Bayreuth, Professor Kessler's team has been researching for years to unveil the secret of spider silk.
How do spiders manage to first store the silk proteins in the silk gland and to then assemble them in the spinning passage in a split second to form threads with these extraordinary characteristics? And what exactly gives the threads their tremendous tensile strength? Scientists have now come one step closer to answering these key questions for the production of artificial spider silk.
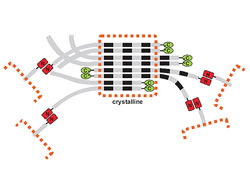
Spider threads consist of long chains of thousands of repeating sequences of protein molecules. These silk proteins are stored in the silk gland in a highly concentrated form until they are needed. The long chains with their repeating sequences of protein molecules are initially unordered and must not get too close to each other as they would immediately clump up. Only in the spinning passage, just before being used, are the threads oriented parallel to each other and form so-called micro crystallites that are, in turn, assembled to stable threads with cross links.
During the last year, the scientists in Kessler's and Scheibel's team investigated the common garden spider (“cross spider”) to discover the mechanism behind the transition from individual spider silk molecules to connected treads: The individual spider silk proteins are first stored in the silk gland in small drops called micelles.
The scientists identified the regulating element that is responsible for assembling a strong thread from the individual parts. It is the so-called C terminal domain of the silk protein. It prevents the formation of threads in the silk duct with its strong salt concentrations. In the spinning passage, however, where the salt concentration is low and sheer forces are abundant, this domain becomes instable and “sticky.” This causes the chains to overlap and a strong spider silk thread is formed. The discovery of the significance of this relatively small C terminal domain, when compared to the overall length of the protein thread, was a sensation at the time and was published in the renowned scientific journal Nature.
Now the same group of researchers has put in place a further piece in the spider silk puzzle. They showed that the other end of the long thread, the so-called N terminal domain, plays an important role in the design of strong threads with great tensile strength. This time, the scientists investigated the head ends of the spider silk proteins of the “black widow” (Latrodectus hesperus). The result: The N terminal head ends exist in the silk duct as single strands (momomers). Only in the sinning passage are the head to tail pairs (dimers) formed.
The process of laying together is regulated via the change in pH values and salt concentrations between the silk duct and the spinning canal. In the silk duct, a neutral pH value of 7.2 and a high salt concentration prevent the N terminal head ends from combining. In the spinning passage, however, the environment becomes acidic (pH value around 6.2) the salt is removed. Now the ends can come together. In this process, the N terminal ends connect to the respective other ends – a practically endless chain of linked up spider silk proteins is formed. “In our work we were able to show, in addition to our previous research, that both the pH value and the salt concentration influence the monomer-dimer balance,” says Franz Hagen, corresponding author of the study, in summing up the results. “Both factors influence the formation of dimers and thus the efficient cross-linking to very long silk proteins.”
Ultimately, this cross-linking is what gives the spider silk threads their enormous tensile strength. The small crystallites first formed in the parallel cross-linking of the protein chains following the controlled unfolding of the C terminal domain are connected to each other via the N terminal domains of the spider silk protein to form a very long chain. “This is the effect that eventually explains the enormous tensile strength of the spider silk thread,” says Kessler. To date, this ingenious form of cross-linking – called “multivalence” – has not been implemented in artificial polymers. “Most polymer chemists focus on the length of the thread. So far, no one has come up with the approach of cross-linking the ends of the threads and thereby opening the door to virtually unlimited lengths of polymer chains,” beleives Kessler. These new findings may provide chemists with a model for manufacturing new materials with improved characteristics.
The scientists used the method of nuclear magnetic resonance (NMR) to analyze the structure of spider silk. Segments of spider silk are dissolved under conditions similar to those found in spider organs and exposed to radio wave impulses in a very strong magnetic field. The scientists can deduce the exact molecular structure from the “response” of the molecules. Using this method, environmental influences (e.g. salt concentration and pH value) can be studied accurately under simulated natural conditions. The development and application of NMR methods to biomolecules has been a longstanding focus of the Bavarian NMR Center in Garching.
Media Contact
More Information:
http://www.tum.deAll latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

Silicon Carbide Innovation Alliance to drive industrial-scale semiconductor work
Known for its ability to withstand extreme environments and high voltages, silicon carbide (SiC) is a semiconducting material made up of silicon and carbon atoms arranged into crystals that is…

New SPECT/CT technique shows impressive biomarker identification
…offers increased access for prostate cancer patients. A novel SPECT/CT acquisition method can accurately detect radiopharmaceutical biodistribution in a convenient manner for prostate cancer patients, opening the door for more…

How 3D printers can give robots a soft touch
Soft skin coverings and touch sensors have emerged as a promising feature for robots that are both safer and more intuitive for human interaction, but they are expensive and difficult…





















