Carnegie Mellon University creates novel carbon nanoparticles with vast potential
Innovative polymer chemistry employed
Carnegie Mellon University scientists have developed an attractive way to make discrete carbon nanoparticles for electrical components used in industry and research. This method, which employs polyacrylonitrile (PAN) as a nanoparticle precursor, is being presented by Chuanbing Tang, a Carnegie Mellon graduate student, on Sunday, March 28, at the 227th annual meeting of the American Chemical Society in Anaheim, Calif. (POLY69, Garden A). The research findings have been accepted for publication in Angewandte Chemie, International Edition.
“This work really illustrates a particularly attractive strategy in the evolution of nanotechnology,” said Tomasz Kowalewski, assistant professor of chemistry at the Mellon College of Science and principal investigator on this research, which is supported by the National Science Foundation. “Our well-defined carbon nanoparticles should find a wide range of applications, especially in energy storage/conversion devices and in display technologies.”
The Carnegie Mellon group is currently working on using carbon nanoparticles as active materials in field emitter arrays for flat panel screen displays. This technology to produce carbon nanostructures also could be adapted to produce solar panels that convert sunlight into electrical energy. Other applications include the development of carbon-based nanosensors or high-surface area electrodes for use in biotechnology or medicine.
The Carnegie Mellon approach is relatively low cost, simple and potentially scalable to commercial production levels, said Kowalewski, who added these are significant advantages over existing technologies to make well-defined nanostructured carbons. Using the method, PAN copolymers serving as carbon precursors can be deposited as thin films on surfaces (e.g. silicon wafers), where they can be patterned and further processed using techniques currently employed to fabricate microelectronic devices. Such a seamless manufacturing process is important to generate integrated devices and would be difficult to achieve with other methods currently used to synthesize nanostructured carbons, said Kowalewski.
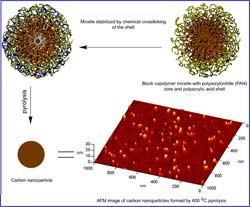
The new approach is based on a method the Carnegie Mellon group previously developed to form nanostructured carbons by using block copolymers in which PAN is linked to other polymers with which it normally does not mix. In the current method, PAN, a “water-hating” compound, is copolymerized with polyacrylic acid, a “water-loving” polymer. In water-containing solutions, PAN-polyacrylic acid copolymers self assemble into nanoscale droplets, or micelles. Each micelle has a water-insoluble PAN core and a water-soluble polyacrylic acid outer coat that forms an outer shell.
To make carbon nanoparticles from micelles, the Carnegie Mellon scientists used a shell-crosslinking technique developed by team collaborator Karen Wooley, a chemist at Washington University in St. Louis. The scientists then deposited thin and ultra-thin films of these nanoparticles on various substrates. Per their previously developed method, the Carnegie Mellon team heated the nanoparticles to high temperatures in a process called pyrolysis. This step decomposed the polyacrylic acid shell scaffolding and converted the chemically stabilized PAN domains into arrays of discrete carbon nanostructures. (See figure.)
“Self assembly of copolymers can be used to pre-organize them into a variety of nanostructures for many uses,” said Kowalewski. Self-assembly of block copolymers is closely related to a familiar process of phase separation of immiscible fluids (e.g., oil and water). But unlike oil and water, immiscible blocks in a copolymer are chemically linked to each other so that phase separation domains lie within a few tens of nanometers of each other. Previously, Kowalewski’s group used self assembly of PAN-containing copolymers in the bulk followed by their pyrolysis to produce arrays of carbon nanoclusters.
The Carnegie Mellon investigators used various controlled radical polymerization (CRP) methods – including one (atom radical transfer polymerization) developed by Krzysztof Matyjaszewski at Carnegie Mellon – to create their structures. CRP allows precise control of the growth of each polymer chain and can be used to extend one type of polymer chain with a different type of polymer, resulting in block copolymers. Atomic force microscopy and spectroscopic studies have shown that the Carnegie Mellon-manufactured copolymers produce well organized carbon nanostructures.
The Mellon College of Science is one of the nation’s leading innovators in polymer chemistry. For more information, please visit www.chem.cmu.edu/groups/kowalewski.
Media Contact
All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















