Phagraphene, a 'relative' of graphene, discovered
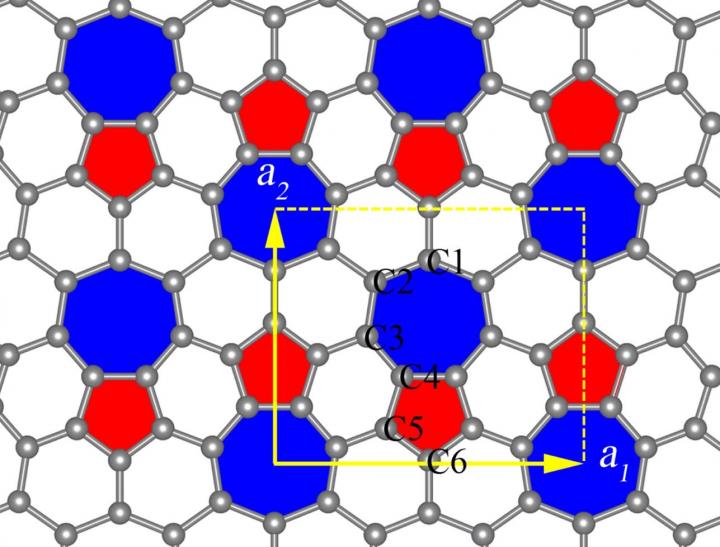
This is a phagraphene structure. Credit: Artyom Oganov
“Unlike graphene, a hexagonal honeycomb structure with atoms of carbon at its junctions, phagraphene consists of penta-, hexa- and heptagonal carbon rings. Its name comes from a contraction of Penta-Hexa-heptA-graphene,” says Oganov, head of the MIPT Laboratory of Computer Design.
Two-dimensional materials, composed of a one-atom-thick layer, have attracted great attention from scientists in the last few decades. The first of these materials, graphene, was discovered in 2004 by two MIPT graduates, Andre Geim and Konstantin Novoselov. In 2010 Geim and Novoselov were awarded the Nobel Prize in physics for that achievement.
Due to its two-dimensional structure, graphene has absolutely unique properties. Most materials can transmit electric current when unbound electrons have an energy that corresponds to the conduction band of the material.
When there is a gap between the range of possible electron energies, the valence band, and the range of conductivity (the so-called forbidden zone), the material acts as an insulator. When the valence band and conduction band overlap, it acts a conductor, and electrons can move under the influence of electric field.
In graphene each carbon atom has three electrons that are bound to electrons in neighboring atoms, forming chemical bonds. The fourth electron of each atom is “delocalized” throughout the whole graphene sheet, which allows it to conduct electrical current.
At the same time, the forbidden zone in the graphene has zero width. If you plot the electron energy and their location in graph form, you get a figure resembling an hour glass, i.e. two cones connected by vertices. These are known as Dirac cones.
Due to this unique condition, electrons in graphene behave very strangely: all of them have one and the same velocity (which is comparable to the velocity of light), and they possess no inertia. They appear to have no mass.
And, according to the theory of relativity, particles traveling at the velocity of light must behave in this manner. The velocity of electrons in graphene is about 10 thousand kilometers a second (electron velocities in a typical conductor vary from centimeters up to hundreds of meters per second).
Phagraphene, discovered by Oganov and his colleagues through the use of the USPEX algorithm, as well as graphene, is a material where Dirac cones appear, and electrons behave similar to particles without mass.
“In phagraphene, due to the different number of atoms in the rings, the Dirac cones are 'inclined.' That is why the velocity of electrons in it depends on the direction. This is not the case in graphene. It would be very interesting for future practical use to see where it will be useful to vary the electron velocity,” Artyom Oganov explains.
Phagraphene possesses all the other properties of graphene that allows it to be considered an advanced material for flexible electronic devices, transistors, solar batteries, display units and many other things.
###
Phagraphene: A Low-Energy Graphene Allotrope Composed of 5-6-7 Carbon Rings with Distorted Dirac Cones
DOI: 10.1021/acs.nanolett.5b02512
Media Contact
All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

Bringing bio-inspired robots to life
Nebraska researcher Eric Markvicka gets NSF CAREER Award to pursue manufacture of novel materials for soft robotics and stretchable electronics. Engineers are increasingly eager to develop robots that mimic the…

Bella moths use poison to attract mates
Scientists are closer to finding out how. Pyrrolizidine alkaloids are as bitter and toxic as they are hard to pronounce. They’re produced by several different types of plants and are…

AI tool creates ‘synthetic’ images of cells
…for enhanced microscopy analysis. Observing individual cells through microscopes can reveal a range of important cell biological phenomena that frequently play a role in human diseases, but the process of…





















