Efficient Production Process for Coveted Nanocrystals
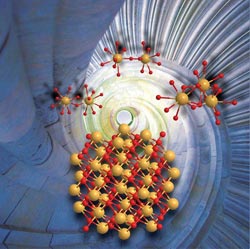
Ce(IV) dimers and trimers form in aqueous solution nanometer-sized cer dioxide crystals (CeO2). The size of the nanocrystals is in the order of two to three nanometers.<br>Picture: A. Ikeda-Ohno<br>
The research results were published in the scientific journal Chemistry – A European Journal (DOI: 10.1002/chem.201204101). This finding potentially simplifies and alleviates the existing synthetic processes of nanocrystalline CeO2 production.
Nanocrystalline CeO2 particles are widely used, for example, in catalysts for hazardous gas treatment, in electrodes for solid oxide fuel cells, in polishing materials for advanced integrated circuits, in sunscreen cosmetics, and in such medical applications as artificial superoxide dismutase. Current industrial syntheses of nanocrystalline CeO2 are based on sol-gel processes followed by thermal treatment and/or the addition of accelerant reagents. Any further improvement of the synthetic strategy for CeO2 nanocrystals requires a better understanding of the mechanisms involved in their formation at the atomic scale.
Dr. Atsushi Ikeda-Ohno from the University of New South Wales, Australia, together with Dr. Christoph Hennig from the HZDR opted for a sophisticated multi-spectroscopic approach that combines dynamic light scattering and synchrotron-based X-ray techniques. These complex investigations involved the use of two world-leading synchrotron facilities of the European Synchrotron Radiation Facility (ESRF) in Grenoble, France, and SPring-8 in Hyogo, Japan.
Live Monitoring
For the first time ever, the scientists were able to perform an in-situ observation of nanocrystal evolution. So far, little has been known of the formation mechanism of metal nanocrystals; mainly because appropriate analytical techniques were lacking. The most widely used techniques for metal nanocrystal research are electron microscopy and X-ray diffraction. They are powerful enough to visualize the appearance of nanocrystals and to acquire their lattice information, but they are not applicable to the solution state where the evolution of metal nanocrystals occurs. “To probe the formation of nanocrystalline CeO2 in an aqueous solution, we combined different spectroscopic techniques, including dynamic light scattering, synchrotron X-ray absorption spectroscopy, and high energy X-ray scattering,” says Dr. Atsushi Ikeda-Ohno.
The information the researchers obtained is fundamental to simplifying and alleviating the synthetic process of CeO2 nanocrystals. They revealed that uniformly sized nanoparticles of CeO2 can be produced simply by pH adjustment of tetravalent cerium (Ce(IV)) in an aqueous solution without subsequent physical/chemical treatment such as heating or adding accelerant chemicals. The produced CeO2 crystals have a uniform particle size of 2 – 3 nanometers, irrespective of the preparation conditions (e.g. pH and type of pH adjustment). This particle size is exactly in the range which is interesting for industrial applications. A key finding is that mononuclear Ce(IV) solution species do not result in nano-sized CeO2 crystals. The prerequisite is the presence of oligomeric Ce(IV) solution species, such as dimers or trimers.
“We’re indeed very glad that our multi-spectroscopic approach is also applicable to any other research on metal nanocrystals. That’s why this study contributes to an emerging research area on metal nanocrystals in a broader context,” says Dr. Christoph Hennig. “And the HZDR’s own measuring station at the ESRF provides the best possible opportunities for this research area of metal nanocrystals which directly contributes to industrial applications.”
Publication:
A. Ikeda-Ohno et al., Chem. Eur. J., 19(23), 7348-7360 (2013), DOI: 10.1002/chem.201204101.
Further Information:
Dr. Atsushi Ikeda-Ohno
School of Civil and Environmental Engineering
The University of New South Wales
UNSW, Sydney, New South Wales 2052, Australia
Phone: +61 2 9385 0128
a.ikeda@unsw.edu.au
Dr. Christoph Hennig | Dr. Vinzenz Brendler
Institute of Ressource Ecology at HZDR
Rossendorf Beamline at the ESRF/Grenoble
Phone: +33 476 88 – 2005 | +49 351 260 – 3210
hennig@esrf.fr | v.brendler@hzdr.de
Media Contact:
Dr. Christine Bohnet
Press Officer
Phone: +49 351-260 2450 or +49 160 969 288 56 | c.bohnet@hzdr.de | www.hzdr.de
Helmholtz-Zentrum Dresden-Rossendorf | Bautzner Landstr. 400 | 01328 Dresden | Germany
The Helmholtz-Zentrum Dresden-Rossendorf (HZDR) conducts research in the sectors energy, health, and matter. It focuses its research on the following topics:
• How can energy and resources be used efficiently, safely, and sustainably?
• How can malignant tumors be visualized and characterized more precisely and treated effectively?
• How do matter and materials behave in strong fields and at the smallest dimensions?
To answer these scientific questions, five large-scale research facilities provide unique research opportunities. These facilities are also accessible to external users.
The HZDR has been a member of the Helmholtz Association, Germany’s largest research organization, since 2011. It has four locations in Dresden, Leipzig, Freiberg, and Grenoble and employs about 1,000 people – approx. 450 of whom are scientists including 160 doctoral candidates.
Media Contact
More Information:
http://www.hzdr.deAll latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

Bringing bio-inspired robots to life
Nebraska researcher Eric Markvicka gets NSF CAREER Award to pursue manufacture of novel materials for soft robotics and stretchable electronics. Engineers are increasingly eager to develop robots that mimic the…

Bella moths use poison to attract mates
Scientists are closer to finding out how. Pyrrolizidine alkaloids are as bitter and toxic as they are hard to pronounce. They’re produced by several different types of plants and are…

AI tool creates ‘synthetic’ images of cells
…for enhanced microscopy analysis. Observing individual cells through microscopes can reveal a range of important cell biological phenomena that frequently play a role in human diseases, but the process of…





















