3D printed, bioinspired heart valves
The Chair of Medical Materials and Implants (MMI) is following different strategies to combine a technical component, which provides structural stability, with a biological one to obtain living tissue/organs that recapitulate the mechanical and biological functions of the healthy equivalents.
Copyright: Andreas Heddergott / TUM
Researchers have developed 3D printed artificial heart valves designed to allow a patient’s own cells to form new tissue.
To form these scaffolds using melt electrowriting – an advanced additive manufacturing technique – the team has created a new fabrication platform that enables them to combine different precise, customized patterns and hence to fine-tune the scaffold’s mechanical properties. Their long-term goal is to create implants for children that develop into new tissue and therefore last a lifetime.
In the human body, four heart valves ensure that blood flows in the correct direction. It is essential that heart valves open and close properly. To fulfil this function, heart valve tissue is heterogeneous, meaning that heart valves display different biomechanical properties within the same tissue.
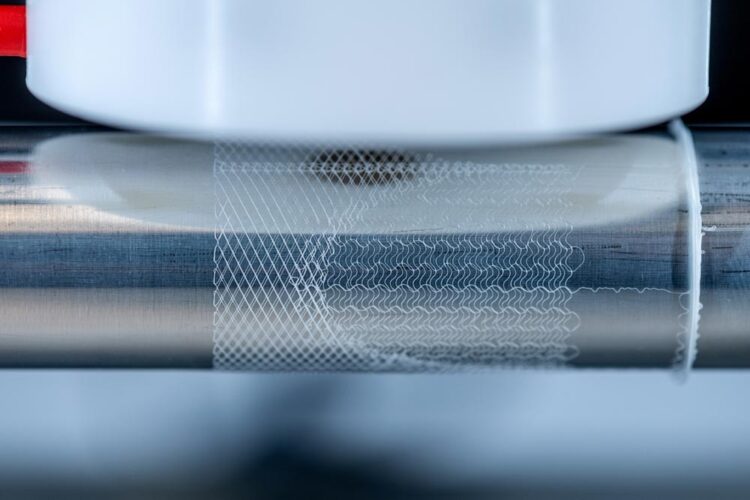
A team of researchers working with Petra Mela, Professor of Medical Materials and Implants at the Technical University of Munich (TUM), and Professor Elena De-Juan Pardo from The University of Western Australia, have now, for the first time, imitated this heterogeneous structure using a 3D printing process called melt electrowriting. To do this, they have developed a platform that facilitates printing precise customized patterns and their combination, which enabled them to fine-tune different mechanical properties within the same scaffold.
Melt electrowriting enables the creation of precise and customized fiber scaffolds
Melt electrowriting is a comparatively new additive manufacturing technology that uses high voltage to create accurate patterns of very thin polymer fibers. A polymer is heated, melted and pushed out of a printing head as a liquid jet to form the fibers.
During this process, a high-voltage electric field is applied, which considerably narrows the diameter of the polymer jet by accelerating it and pulling it towards a collector. This results in a thin fiber with a diameter typically in the range of five to fifty micrometers. Moreover, the electric field stabilizes the polymer jet, which is important for creating defined, precise patterns.
The “writing” of the fiber jet according to predefined patterns is conducted using a computer-controlled moving collector. Similar to moving a slice of bread below a spoon dripping with honey, the moving platform collects the emerging fiber along a defined pathway. The user specifies this pathway by programming its coordinates.
In order to considerably reduce the programming effort associated with the creation of complex structures for heart valves, the researchers developed a software to easily assign different patterns to different regions of the scaffold by choosing from a library of available patterns. Furthermore, geometrical specifications such as the length, diameter and thickness of the scaffold can easily be adjusted via the graphical interface.
The heart valve scaffolds are compatible with cells and biodegradable
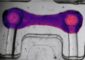
The team used medical grade polycaprolactone (PCL) for 3D printing, which is compatible with cells and biodegradable. The idea is that once the PCL-heart valves are implanted, the patient’s own cells will grow on the porous scaffold, as has been the case in first cell culture studies. The cells might then potentially form new tissue, before the PCL-scaffold degrades.
The PCL-scaffold is embedded in an elastin-like material that imitates properties of natural elastin present in real heart valves and provides micro-pores smaller than the pores of the PCL structure. The aim is to leave enough space for the cells to settle, but to seal the valves adequately for blood flow.
The engineered valves were tested using a mock flow circulatory system simulating physiological blood pressure and flow. The heart valves opened and closed correctly under the examined conditions.
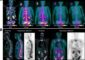
Nanoparticles allow for visualization using MRI
The PCL-material was further evolved and evaluated together with Franz Schilling, Professor of Biomedical Magnetic Resonance, and Sonja Berensmeier, Professor of Bioseparation Engineering at TUM. By modifying PCL with ultrasmall superparamagnetic iron oxide nanoparticles, the researchers could visualize the scaffolds using magnetic resonance imaging (MRI). The modified material remains printable and compatible with cells. This might facilitate the translation of the technology to the clinics, as the scaffolds can thus be monitored upon implantation.
“Our goal is to engineer bioinspired heart valves that support the formation of new functional tissue in patients. Children would especially benefit from such a solution, as current heart valves do not grow with the patient and therefore have to be replaced over the years in multiple surgeries. Our heart valves, in contrast, mimic the complexity of native heart valves and are designed to let a patient’s own cells infiltrate the scaffold”, says Petra Mela.
The next step on the way to the clinic will be pre-clinical studies in animal models. The team also works on further improving the technology and developing new biomaterials.
Wissenschaftliche Ansprechpartner:
Prof. Dr. Petra Mela
Technical University of Munich
Chair of Medical Materials and Implants
Phone: +49 (0)89 289 16701
petra.mela@tum.de
https://www.mec.ed.tum.de/en/mmi/home/
Originalpublikation:
Saidy, N. T., Fernández-Colino, A., Heidari, B. S., Kent, R., Vernon, M., Bas, O., Mulderrig, S., Lubig, A., Rodríguez-Cabello, J. C., Doyle, B., Hutmacher, D. W., De-Juan-Pardo, E. M., Mela, P., Spatially Heterogeneous Tubular Scaffolds for In Situ Heart Valve Tissue Engineering Using Melt Electrowriting. Adv. Funct. Mater. 2022, 2110716. https://doi.org/10.1002/adfm.202110716
Mueller, K. M. A., Topping, G. J., Schwaminger, S. P., Zou, Y., Rojas-González, D. M., De.Juan-Pardo, E., Berensmeier, S., Schilling, F., Mela, P., Visualization of USPIO-labeled melt-electrowritten scaffolds by non-invasive magnetic resonance imaging, Biomater. Sci. 2021, 9. https://doi.org/10.1039/D1BM00461A
Weitere Informationen:
https://mediatum.ub.tum.de/1659641 High resolution images
https://www.bioengineering.tum.de/en/ Munich Institute of Biomedical Engineering
https://www.tum.de/en/about-tum/news/press-releases/details/37446
Media Contact
All latest news from the category: Medical Engineering
The development of medical equipment, products and technical procedures is characterized by high research and development costs in a variety of fields related to the study of human medicine.
innovations-report provides informative and stimulating reports and articles on topics ranging from imaging processes, cell and tissue techniques, optical techniques, implants, orthopedic aids, clinical and medical office equipment, dialysis systems and x-ray/radiation monitoring devices to endoscopy, ultrasound, surgical techniques, and dental materials.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…